Rivastigmine-Bambuterol Hybrids as Selective Butyrylcholinesterase Inhibitors
- PMID: 38202655
- PMCID: PMC10780165
- DOI: 10.3390/molecules29010072
Rivastigmine-Bambuterol Hybrids as Selective Butyrylcholinesterase Inhibitors
Abstract
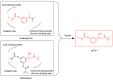
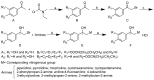
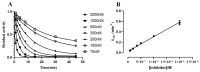
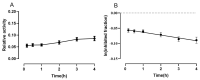
Selective butyrylcholinesterase inhibitors are considered promising drug candidates for the treatment of Alzheimer's disease. In this work, one rivastigmine-bambuterol hybrid (MTR-1) and fourteen of its analogues were synthesized, purified, and characterized. In vitro cholinesterase assays showed that all the compounds were more potent inhibitors of BChE when compared to AChE. Further investigations indicated that MTR-3 (IC50(AChE) > 100,000 nM, IC50(BChE) = 78 nM) was the best compound in the series, showing high butyrylcholinesterase selectivity and inhibition potency, the potential to permeate the blood-brain barrier, and longer-lasting BChE inhibition than bambuterol. These compounds could be used to discover novel specific BChE inhibitors for the treatment of Alzheimer's disease.
Keywords: Alzheimer’s disease; bambuterol; butyrylcholinesterase; hybrid; rivastigmine.
Conflict of interest statement
Author Wen Tan was employed by the company Post-Doctoral Innovation Site, Jinan University Affiliation, Yuanzhi Health-Tech Inc. and Kesi (Shandong) Innovation Service Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Identification of Compounds for Butyrylcholinesterase Inhibition.SLAS Discov. 2021 Dec;26(10):1355-1364. doi: 10.1177/24725552211030897. Epub 2021 Jul 16. SLAS Discov. 2021. PMID: 34269114 Free PMC article.
-
Design, synthesis and biological evaluation of novel carbamates as potential inhibitors of acetylcholinesterase and butyrylcholinesterase.Bioorg Med Chem. 2020 Mar 1;28(5):115324. doi: 10.1016/j.bmc.2020.115324. Epub 2020 Jan 18. Bioorg Med Chem. 2020. PMID: 32008882
-
Amino acid residues involved in the interaction of acetylcholinesterase and butyrylcholinesterase with the carbamates Ro 02-0683 and bambuterol, and with terbutaline.Biochim Biophys Acta. 1999 Aug 17;1433(1-2):261-71. doi: 10.1016/s0167-4838(99)00124-7. Biochim Biophys Acta. 1999. PMID: 10446376
-
Butyrylcholinesterase Protein Ends in the Pathogenesis of Alzheimer's Disease-Could BCHE Genotyping Be Helpful in Alzheimer's Therapy?Biomolecules. 2019 Oct 9;9(10):592. doi: 10.3390/biom9100592. Biomolecules. 2019. PMID: 31601022 Free PMC article. Review.
-
Rivastigmine: the advantages of dual inhibition of acetylcholinesterase and butyrylcholinesterase and its role in subcortical vascular dementia and Parkinson's disease dementia.Clin Interv Aging. 2017 Apr 18;12:697-707. doi: 10.2147/CIA.S129145. eCollection 2017. Clin Interv Aging. 2017. PMID: 28458525 Free PMC article. Review.
Cited by
-
Implication of Central β2 Adrenergic Receptor for the Development of Novel Drugs Against Alzheimer's Disease.Arch Pharm (Weinheim). 2025 Apr;358(4):e2400750. doi: 10.1002/ardp.202400750. Arch Pharm (Weinheim). 2025. PMID: 40170395 Free PMC article. Review.
References
-
- Abedinifar F., Farnia S.M.F., Mahdavi M., Nadri H., Moradi A., Ghasemi J.B., Küçükkılınç T.T., Firoozpour L., Foroumadi A. Synthesis and cholinesterase inhibitory activity of new 2-benzofuran carboxamide-benzylpyridinum salts. Bioorganic Chem. 2018;80:180–188. doi: 10.1016/j.bioorg.2018.06.006. - DOI - PubMed
-
- Ghobadian R., Nadri H., Moradi A., Bukhari S.N.A., Mahdavi M., Asadi M., Akbarzadeh T., Khaleghzadeh-Ahangar H., Sharifzadeh M., Amini M. Design, synthesis, and biological evaluation of selective and potent Carbazole-based butyrylcholinesterase inhibitors. Bioorganic Med. Chem. 2018;26:4952–4962. doi: 10.1016/j.bmc.2018.08.035. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

