Search for Osme Bonds with π Systems as Electron Donors
- PMID: 38202661
- PMCID: PMC10779769
- DOI: 10.3390/molecules29010079
Search for Osme Bonds with π Systems as Electron Donors
Abstract
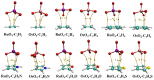
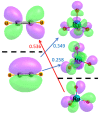
The Osme bond is defined as pairing a Group 8 metal atom as an electron acceptor in a noncovalent interaction with a nucleophile. DFT calculations with the ωB97XD functional consider MO4 (M = Ru, Os) as the Lewis acid, paired with a series of π electron donors C2H2, C2H4, C6H6, C4H5N, C4H4O, and C4H4S. The calculations establish interaction energies in the range between 9.5 and 26.4 kJ/mol. Os engages in stronger interactions than does Ru, and those involving more extensive π-systems within the aromatic rings form stronger bonds than do the smaller ethylene and acetylene. Extensive analysis questions the existence of a true Osme bond, as the bonding chiefly involves interactions with the three O atoms of MO4 that lie closest to the π-system, via π(C-C)→σ*(M-O) transfers. These interactions are supplemented by back donation from M-O bonds to the π*(CC) antibonding orbitals of the π-systems. Dispersion makes a large contribution to these interactions, higher than electrostatics and much greater than induction.
Keywords: AIM; NBO; SAPT; back donation; σ-hole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

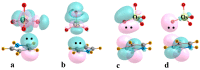
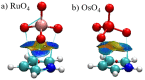

Similar articles
-
Wolfium Bonds with π Systems as Electron Donors.Chemphyschem. 2025 May 5;26(9):e202401095. doi: 10.1002/cphc.202401095. Epub 2025 Apr 4. Chemphyschem. 2025. PMID: 39952896
-
Triel Bonds, π-Hole-π-Electrons Interactions in Complexes of Boron and Aluminium Trihalides and Trihydrides with Acetylene and Ethylene.Molecules. 2015 Jun 19;20(6):11297-316. doi: 10.3390/molecules200611297. Molecules. 2015. PMID: 26102066 Free PMC article.
-
Sigma-hole carbon-bonding interactions in carbon-carbon double bonds: an unnoticed contact.Phys Chem Chem Phys. 2017 Jun 14;19(23):15530-15540. doi: 10.1039/c7cp01780d. Phys Chem Chem Phys. 2017. PMID: 28581553
-
Gold(III) derivatives as the noncovalent interaction donors: theoretical study of the π-hole regium bonds.Phys Chem Chem Phys. 2023 Nov 1;25(42):29155-29164. doi: 10.1039/d3cp04354a. Phys Chem Chem Phys. 2023. PMID: 37870082
-
Electrostatics and Polarization in σ- and π-Hole Noncovalent Interactions: An Overview.Chemphyschem. 2020 Apr 2;21(7):579-588. doi: 10.1002/cphc.201900968. Epub 2020 Jan 16. Chemphyschem. 2020. PMID: 31733136 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

