Recovering Phosphate from Complex Wastewater Using Macroporous Cryogel Composited Calcium Silicate Hydrate Nanoparticles
- PMID: 38202812
- PMCID: PMC10780374
- DOI: 10.3390/molecules29010228
Recovering Phosphate from Complex Wastewater Using Macroporous Cryogel Composited Calcium Silicate Hydrate Nanoparticles
Abstract
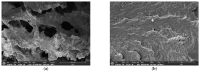
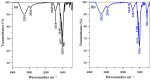
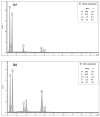
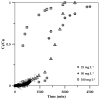
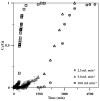
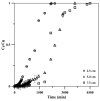
Since currently used natural, nonrenewable phosphorus resources are estimated to be depleted in the next 30-200 years, phosphorus recovery from any phosphorus-rich residues has attracted great interest. In this study, phosphorus recovery from complex wastewater samples was investigated using continuous adsorption on cryogel column composited calcium silicate hydrate nanoparticles (CSH columns). The results showed that 99.99% of phosphate was recovered from a synthetic water sample (50 mg L-1) using a 5 cm CSH column with a 5 mL min-1 influent flow rate for 6 h while 82.82% and 97.58% of phosphate were recovered from household laundry wastewater (1.84 mg L-1) and reverse osmosis concentrate (26.46 mg L-1), respectively. The adsorption capacity decreased with an increasing flow rate but increased with increasing initial concentration and column height, and the obtained experimental data were better fitted to the Yoon-Nelson model (R2 = 0.7723-0.9643) than to the Adams-Bohart model (R2 = 0.6320-0.8899). The adsorption performance of phosphate was decreased 3.65 times in the presence of carbonate ions at a similar concentration, whereas no effect was obtained from nitrate and sulfate. The results demonstrate the potential of continuous-flow phosphate adsorption on the CSH column for the recovery of phosphate from complex wastewater samples.
Keywords: calcium silicate hydrate; landry wastewater; phosphate recovery; phosphate removal; reverse osmosis concentrate; starch cryogel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Continuous Phosphate Removal and Recovery Using a Calcium Silicate Hydrate Composite Monolithic Cryogel Column.Polymers (Basel). 2023 Jan 20;15(3):539. doi: 10.3390/polym15030539. Polymers (Basel). 2023. PMID: 36771839 Free PMC article.
-
Removal and recovery of phosphate using a novel calcium silicate hydrate composite starch cryogel.J Environ Manage. 2022 Jan 1;301:113923. doi: 10.1016/j.jenvman.2021.113923. Epub 2021 Oct 8. J Environ Manage. 2022. PMID: 34634722
-
Calcium silicate hydrate complex konjac glucomannan-based hydrogel selectively adsorbed phosphate in low alkalinity solution.J Environ Manage. 2024 Nov;370:122560. doi: 10.1016/j.jenvman.2024.122560. Epub 2024 Sep 19. J Environ Manage. 2024. PMID: 39299108
-
Continuous-Flow System for Methylene Blue Removal Using a Green and Cost-Effective Starch Single-Rod Column.Polymers (Basel). 2023 Oct 4;15(19):3989. doi: 10.3390/polym15193989. Polymers (Basel). 2023. PMID: 37836037 Free PMC article.
-
Recovery of Phosphate(V) Ions from Water and Wastewater Using Chitosan-Based Sorbents Modified-A Literature Review.Int J Mol Sci. 2023 Jul 27;24(15):12060. doi: 10.3390/ijms241512060. Int J Mol Sci. 2023. PMID: 37569435 Free PMC article. Review.
Cited by
-
Continuous-flow phosphate removal using Cry-Ca-COS Monolith: Insights from dynamic adsorption modeling.Water Res X. 2024 Dec 13;27:100296. doi: 10.1016/j.wroa.2024.100296. eCollection 2025 May 1. Water Res X. 2024. PMID: 39811253 Free PMC article.
References
-
- Geissler B., Mew M.C., Weber O., Steiner G. Efficiency performance of the world’s leading corporations in phosphate rock mining. Resour. Conserv. Recycl. 2015;105:246–258. doi: 10.1016/j.resconrec.2015.10.008. - DOI
-
- Cordell D., White S. Tracking phosphorus security: Indicators of phosphorus vulnerability in the global food system. Food Secur. 2015;7:337–350. doi: 10.1007/s12571-015-0442-0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

