Pleiotropic Potential of Evernia prunastri Extracts and Their Main Compounds Evernic Acid and Atranorin: In Vitro and In Silico Studies
- PMID: 38202817
- PMCID: PMC10780513
- DOI: 10.3390/molecules29010233
Pleiotropic Potential of Evernia prunastri Extracts and Their Main Compounds Evernic Acid and Atranorin: In Vitro and In Silico Studies
Abstract
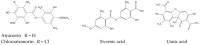
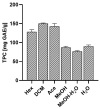
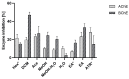
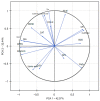
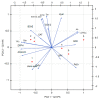
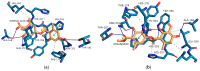
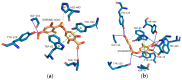
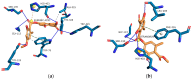
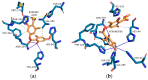
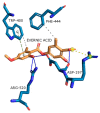
Evernia prunastri is a lichen widely distributed in the Northern Hemisphere. Its biological properties still need to be discovered. Therefore, our paper focuses on studies of E. prunastri extracts, including its main metabolites evernic acid (EA) or atranorin (ATR). Phytochemical profiles using chromatographic analysis were confirmed. The antioxidant activity was evaluated using in vitro chemical tests and in vitro enzymatic cells-free tests, namely superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), and catalase (CAT). The anti-inflammatory potential using cyclooxygenase-2 (COX-2) and hyaluronidase were determined. The neuroprotective potential using acetylcholinesterase, (AChE), butyrylcholinesterase (BChE), and tyrosinase (Tyr) was estimated. The hypoglycemic activity was also confirmed (α-glucosidase). Principal component analysis was performed to determine the relationship between the biological activity of extracts. The inhibitory effect of EA and ATR on COX-2 AChE, BChE, Tyr, and α-glucosidase was evaluated using molecular docking techniques and confirmed for EA and ATR (besides α-glucosidase). The penetration of EA and ATR from extracts through the blood-brain barrier was confirmed using the parallel artificial membrane permeability assay blood-brain barrier test. In conclusion, depending on chemical surroundings and the concentration, the E. prunastri extracts, EA or ATR, showed attractive pleiotropic properties, which should be further investigated.
Keywords: enzyme inhibition; lichen; molecular docking; neurodegenerative diseases; oak moss.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

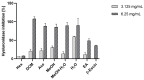
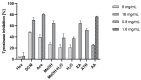
Similar articles
-
Chemical Profiling and In Vitro Evaluation of Bioactive Properties of Evernia prunastri Extract: Implications for Therapeutic Applications.Plants (Basel). 2025 Feb 14;14(4):583. doi: 10.3390/plants14040583. Plants (Basel). 2025. PMID: 40006842 Free PMC article.
-
Phytochemical profile, enzyme inhibition activity and molecular docking analysis of Feijoa sellowiana O. Berg.J Enzyme Inhib Med Chem. 2021 Dec;36(1):618-626. doi: 10.1080/14756366.2021.1880397. J Enzyme Inhib Med Chem. 2021. PMID: 33557639 Free PMC article.
-
Antimicrobial and antioxidant activity of Evernia prunastri extracts and their isolates.World J Microbiol Biotechnol. 2021 Jul 7;37(8):129. doi: 10.1007/s11274-021-03099-y. World J Microbiol Biotechnol. 2021. PMID: 34232401 Free PMC article.
-
Lichen-Derived Compounds and Extracts as Biologically Active Substances with Anticancer and Neuroprotective Properties.Pharmaceuticals (Basel). 2021 Dec 10;14(12):1293. doi: 10.3390/ph14121293. Pharmaceuticals (Basel). 2021. PMID: 34959693 Free PMC article.
-
Unveiling Natural and Semisynthetic Acylated Flavonoids: Chemistry and Biological Actions in the Context of Molecular Docking.Molecules. 2022 Aug 26;27(17):5501. doi: 10.3390/molecules27175501. Molecules. 2022. PMID: 36080269 Free PMC article. Review.
Cited by
-
Prebiotic Systems Containing Anthocyanin-Rich Pomegranate Flower Extracts with Antioxidant and Antidiabetic Effects.Pharmaceutics. 2024 Apr 10;16(4):526. doi: 10.3390/pharmaceutics16040526. Pharmaceutics. 2024. PMID: 38675187 Free PMC article.
-
Evernic Acid: A Low-Toxic and Selective Alternative to Chemotherapeutic Agents in the Treatment of Ovarian Cancer.Arch Pharm (Weinheim). 2025 May;358(5):e70015. doi: 10.1002/ardp.70015. Arch Pharm (Weinheim). 2025. PMID: 40405479 Free PMC article.
-
Cyclodextrin-Based Systems of Cetraria islandica Extracts: A Novel Approach to Improve Solubility and Biological Activity of Lichen-Derived Natural Products.Molecules. 2025 Jul 29;30(15):3182. doi: 10.3390/molecules30153182. Molecules. 2025. PMID: 40807358 Free PMC article.
-
Chemical Profiling and In Vitro Evaluation of Bioactive Properties of Evernia prunastri Extract: Implications for Therapeutic Applications.Plants (Basel). 2025 Feb 14;14(4):583. doi: 10.3390/plants14040583. Plants (Basel). 2025. PMID: 40006842 Free PMC article.
-
Elderberry Leaves with Antioxidant and Anti-Inflammatory Properties as a Valuable Plant Material for Wound Healing.Pharmaceuticals (Basel). 2024 May 10;17(5):618. doi: 10.3390/ph17050618. Pharmaceuticals (Basel). 2024. PMID: 38794188 Free PMC article.
References
-
- Burch J.B., Augustine A.D., Frieden L.A., Hadley E., Howcroft T.K., Johnson R., Khalsa P.S., Kohanski R.A., Li X.L., Macchiarini F. Advances in geroscience: Impact on healthspan and chronic disease. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014;69:S1–S3. doi: 10.1093/gerona/glu041. - DOI - PMC - PubMed
-
- Di Maio A., De Rosa A., Pelucchi S., Garofalo M., Marciano B., Nuzzo T., Gardoni F., Isidori A.M., Di Luca M., Errico F. Analysis of mRNA and protein levels of CAP2, DLG1 and ADAM10 genes in post-mortem brain of schizophrenia, Parkinson’s and Alzheimer’s disease patients. Int. J. Mol. Sci. 2022;23:1539. doi: 10.3390/ijms23031539. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

