Membrane-Disruptive Effects of Fatty Acid and Monoglyceride Mitigants on E. coli Bacteria-Derived Tethered Lipid Bilayers
- PMID: 38202820
- PMCID: PMC10780109
- DOI: 10.3390/molecules29010237
Membrane-Disruptive Effects of Fatty Acid and Monoglyceride Mitigants on E. coli Bacteria-Derived Tethered Lipid Bilayers
Abstract
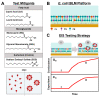
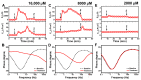
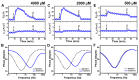
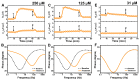
We report electrochemical impedance spectroscopy measurements to characterize the membrane-disruptive properties of medium-chain fatty acid and monoglyceride mitigants interacting with tethered bilayer lipid membrane (tBLM) platforms composed of E. coli bacterial lipid extracts. The tested mitigants included capric acid (CA) and monocaprin (MC) with 10-carbon long hydrocarbon chains, and lauric acid (LA) and glycerol monolaurate (GML) with 12-carbon long hydrocarbon chains. All four mitigants disrupted E. coli tBLM platforms above their respective critical micelle concentration (CMC) values; however, there were marked differences in the extent of membrane disruption. In general, CA and MC caused larger changes in ionic permeability and structural damage, whereas the membrane-disruptive effects of LA and GML were appreciably smaller. Importantly, the distinct magnitudes of permeability changes agreed well with the known antibacterial activity levels of the different mitigants against E. coli, whereby CA and MC are inhibitory and LA and GML are non-inhibitory. Mechanistic insights obtained from the EIS data help to rationalize why CA and MC are more effective than LA and GML at disrupting E. coli membranes, and these measurement capabilities support the potential of utilizing bacterial lipid-derived tethered lipid bilayers for predictive assessment of antibacterial drug candidates and mitigants.
Keywords: antimicrobial; critical micelle concentration; electrochemical impedance spectroscopy; fatty acid; monoglyceride; tethered bilayer lipid membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Mechanistic Evaluation of Antimicrobial Lipid Interactions with Tethered Lipid Bilayers by Electrochemical Impedance Spectroscopy.Sensors (Basel). 2022 May 13;22(10):3712. doi: 10.3390/s22103712. Sensors (Basel). 2022. PMID: 35632121 Free PMC article.
-
Unraveling Membrane-Disruptive Properties of Sodium Lauroyl Lactylate and Its Hydrolytic Products: A QCM-D and EIS Study.Int J Mol Sci. 2023 May 25;24(11):9283. doi: 10.3390/ijms24119283. Int J Mol Sci. 2023. PMID: 37298235 Free PMC article.
-
Unraveling How Antimicrobial Lipid Mixtures Disrupt Virus-Mimicking Lipid Vesicles: A QCM-D Study.Biomimetics (Basel). 2024 Jan 24;9(2):67. doi: 10.3390/biomimetics9020067. Biomimetics (Basel). 2024. PMID: 38392113 Free PMC article.
-
Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications.Int J Mol Sci. 2018 Apr 8;19(4):1114. doi: 10.3390/ijms19041114. Int J Mol Sci. 2018. PMID: 29642500 Free PMC article. Review.
-
Functional tethered lipid bilayers.J Biotechnol. 2000 Sep;74(3):137-58. doi: 10.1016/s1389-0352(00)00012-x. J Biotechnol. 2000. PMID: 11143794 Review.
Cited by
-
Antibacterial potency of acyclic diester, oleic acid, and β-amyrin tetradecanoate from Acacia lahai and Leucas calostachys against antibiotic-resistant bacteria.Front Microbiol. 2025 Jun 25;16:1604820. doi: 10.3389/fmicb.2025.1604820. eCollection 2025. Front Microbiol. 2025. PMID: 40636505 Free PMC article.
-
Valine potentiates cefoperazone-sulbactam to kill methicillin-resistant Staphylococcus aureus.mSystems. 2025 Jan 21;10(1):e0124424. doi: 10.1128/msystems.01244-24. Epub 2024 Dec 18. mSystems. 2025. PMID: 39692510 Free PMC article.
References
-
- Nikoleli G.-P., Siontorou C.G., Nikolelis M.-T., Bratakou S., Bendos D.K. Recent lipid membrane-based biosensing platforms. Appl. Sci. 2019;9:1745. doi: 10.3390/app9091745. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

