The Effect of pH on the Viscoelastic Response of Alginate-Montmorillonite Nanocomposite Hydrogels
- PMID: 38202826
- PMCID: PMC10780325
- DOI: 10.3390/molecules29010244
The Effect of pH on the Viscoelastic Response of Alginate-Montmorillonite Nanocomposite Hydrogels
Abstract
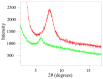
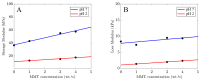
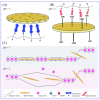
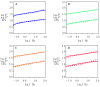
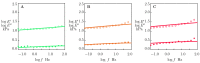
Ionically cross-linked alginate hydrogels are used in a wide range of applications, such as drug delivery, tissue engineering, and food packaging. A shortcoming of these gels is that they lose their strength and degrade at low pH values. To develop gels able to preserve their integrity in a wide range of pH values, Ca-alginate-montmorillonite nanocomposite gels are prepared, and their chemical structure, morphology, and mechanical response are analyzed. As the uniformity of nanocomposite gels is strongly affected by concentrations of MMT and CaCl2, it is revealed that homogeneous gels can be prepared with 4 wt.% MMT and 0.5 M CaCl2 at the highest. The viscoelastic behavior of nanocomposite gels in aqueous solutions with pH = 7 and pH = 2 is investigated by means of small-amplitude compressive oscillatory tests. It is shown that Ca-alginate-MMT nanocomposite gels preserve their integrity while being swollen at pH = 2. The experimental data are fitted by a model with only two material parameters, which shows that the elastic moduli increase linearly with a concentration of MMT at all pH values under investigation due to formation of physical bonds between alginate chains and MMT platelets. The presence of these bonds is confirmed by ATR-FTIR spectroscopy. The morphology of nanocomposite gels is studied by means of wide-angle X-ray diffraction, which reveals that intercalation of polymer chains between clay platelets increases the interlayer gallery spacing.
Keywords: alginate; hydrogel; mechanical properties; montmorillonite; nanocomposite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Singh A.V. Biopolymers in drug delivery: A review. Pharmacologyonline. 2011;1:666–674.
-
- Pires P.C., Mascarenhas-Melo F., Pedrosa K., Lopes D., Lopes J., Macário-Soares A., Peixoto D., Giram P.S., Veiga F., Paiva-Santos A.C. Polymer-based biomaterials for pharmaceutical and biomedical applications: A focus on topical drug administration. Eur. Polym. J. 2023;187:111868. doi: 10.1016/j.eurpolymj.2023.111868. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

