An Overview on Recent Advances in Biomimetic Sensors for the Detection of Perfluoroalkyl Substances
- PMID: 38202993
- PMCID: PMC10781331
- DOI: 10.3390/s24010130
An Overview on Recent Advances in Biomimetic Sensors for the Detection of Perfluoroalkyl Substances
Abstract
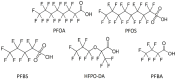
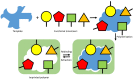
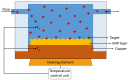
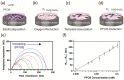
Per- and polyfluoroalkyl substances (PFAS) are a class of materials that have been widely used in the industrial production of a wide range of products. After decades of bioaccumulation in the environment, research has demonstrated that these compounds are toxic and potentially carcinogenic. Therefore, it is essential to map the extent of the problem to be able to remediate it properly in the next few decades. Current state-of-the-art detection platforms, however, are lab based and therefore too expensive and time-consuming for routine screening. Traditional biosensor tests based on, e.g., lateral flow assays may struggle with the low regulatory levels of PFAS (ng/mL), the complexity of environmental matrices and the presence of coexisting chemicals. Therefore, a lot of research effort has been directed towards the development of biomimetic receptors and their implementation into handheld, low-cost sensors. Numerous research groups have developed PFAS sensors based on molecularly imprinted polymers (MIPs), metal-organic frameworks (MOFs) or aptamers. In order to transform these research efforts into tangible devices and implement them into environmental applications, it is necessary to provide an overview of these research efforts. This review aims to provide this overview and critically compare several technologies to each other to provide a recommendation for the direction of future research efforts focused on the development of the next generation of biomimetic PFAS sensors.
Keywords: aptamers; biomimetic sensors; environmental pollution; molecularly imprinted polymers; polyfluoroalkyl substances.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


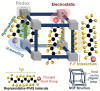
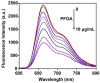
References
-
- Smaili H., Ng C. Adsorption as a Remediation Technology for Short-Chain per- and Polyfluoroalkyl Substances (PFAS) from Water—A Critical Review. Environ. Sci. 2022;9:344–362. doi: 10.1039/D2EW00721E. - DOI
-
- Yu H., Chen Y.F., Guo H.Q., Ma W.T., Li J., Zhou S.G., Lin S., Yan L.S., Li K.X. Preparation of Molecularly Imprinted Carbon Microspheres by One-Pot Hydrothermal Method and Their Adsorption Properties to Perfluorooctane Sulfonate. Chin. J. Anal. Chem. 2019;47:1776–1784. doi: 10.1016/S1872-2040(19)61200-4. - DOI
-
- Hassan M.H., Khan R., Andreescu S. Advances in Electrochemical Detection Methods for Measuring Contaminants of Emerging Concerns. Electrochem. Sci. Adv. 2022;2:e2100184. doi: 10.1002/elsa.202100184. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

