Detection of SO2F2 Using a Photoacoustic Two-Chamber Approach
- PMID: 38203053
- PMCID: PMC10781292
- DOI: 10.3390/s24010191
Detection of SO2F2 Using a Photoacoustic Two-Chamber Approach
Abstract
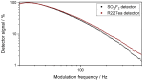
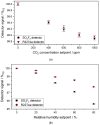
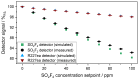
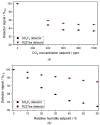
The wide use of sulfuryl difluoride (SO2F2) for termite control in buildings, warehouses and shipping containers requires the implementation of suitable sensors for reliable detection. SO2F2 is highly toxic to humans and the environment, and moreover, it is a potent greenhouse gas. We developed two photoacoustic two-chamber sensors with the aim to detect two different concentration ranges, 0-1 vol.-% SO2F2 and 0-100 ppm SO2F2, so that different applications can be targeted: the sensor for high concentrations for the effective treatment of buildings, containers, etc., and the sensor for low concentrations as personal safety device. Photoacoustic detectors were designed, fabricated, and then filled with either pure SO2F2 or pure substituent gas, the refrigerant R227ea, to detect SO2F2. Absorption cells with optical path lengths of 50 mm and 1.6 m were built for both concentration ranges. The sensitivity to SO2F2 as well as cross-sensitivities to CO2 and H2O were measured. The results show that concentrations below 1 ppm SO2F2 can be reliably detected, and possible cross-sensitivities can be effectively compensated.
Keywords: photoacoustic spectroscopy; sulfuryl difluoride (SO2F2) detection; two-chamber photoacoustic sensors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Yu D., Deng L., Yao B., Vollmer M.K., Chen L., Li Y., Xu H., O’Doherty S., Song Q., Ning S. Atmospheric mixing ratios and emissions of sulfuryl fluoride (SO2F2) in China. Atmos. Res. 2022;275:106222. doi: 10.1016/j.atmosres.2022.106222. - DOI
-
- Gressent A., Rigby M., Ganesan A.L., Prinn R.G., Manning A.J., Mühle J., Salameh P.K., Krummel P.B., Fraser P.J., Steele L.P., et al. Growing atmospheric emissions of sulfuryl fluoride. J. Geophys. Res. Atmos. 2021;126:e2020JD034327. doi: 10.1029/2020JD034327. - DOI
-
- NIOSH Pocket Guide to Chemical Hazards. National Institute for Occupational Safety and Health (NIOSH); Washington, DC, USA: 2019.
-
- Sulbaek Andersen M.P., Blake D.R., Rowland F.S., Hurley M.D., Wallington T.J. Atmospheric chemistry of sulfuryl fluoride: Reaction with OH radicals, Cl atoms and O3, atmospheric lifetime, IR spectrum, and global warming potential. Environ. Sci. Technol. 2009;43:1067–1070. doi: 10.1021/es802439f. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

