Polystyrene Microplastics Exacerbate Candida albicans Infection Ability In Vitro and In Vivo
- PMID: 38203182
- PMCID: PMC10778850
- DOI: 10.3390/ijms25010012
Polystyrene Microplastics Exacerbate Candida albicans Infection Ability In Vitro and In Vivo
Abstract
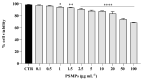
Plastic pollution is an important environmental problem, and microplastics have been shown to have harmful effects on human and animal health, affecting immune and metabolic physiological functions. Further, microplastics can interfere with commensal microorganisms and exert deleterious effects on exposure to pathogens. Here, we compared the effects of 1 µm diameter polystyrene microplastic (PSMPs) on Candida albicans infection in both in vitro and in vivo models by using HT29 cells and Galleria mellonella larvae, respectively. The results demonstrated that PSMPs could promote Candida infection in HT29 cells and larvae of G. mellonella, which show immune responses similar to vertebrates. In this study, we provide new experimental evidence for the risk to human health posed by PSMPs in conjunction with Candida infections.
Keywords: Candida albicans; Galleria mellonella; biofilm; cytokines; polystyrene microplastics; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





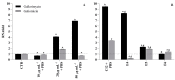

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

