Modulating NO-GC Pathway in Pulmonary Arterial Hypertension
- PMID: 38203205
- PMCID: PMC10779316
- DOI: 10.3390/ijms25010036
Modulating NO-GC Pathway in Pulmonary Arterial Hypertension
Abstract
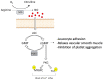
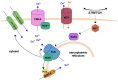
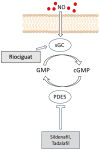
The pathogenesis of complex diseases such as pulmonary arterial hypertension (PAH) is entirely rooted in changes in the expression of some vasoactive factors. These play a significant role in the onset and progression of the disease. Indeed, PAH has been associated with pathophysiologic alterations in vascular function. These are often dictated by increased oxidative stress and impaired modulation of the nitric oxide (NO) pathway. NO reduces the uncontrolled proliferation of vascular smooth muscle cells that leads to occlusion of vessels and an increase in pulmonary vascular resistances, which is the mainstay of PAH development. To date, two classes of NO-pathway modulating drugs are approved for the treatment of PAH: the phosphodiesterase-5 inhibitors (PD5i), sildenafil and tadalafil, and the soluble guanylate cyclase activator (sGC), riociguat. Both drugs provide considerable improvement in exercise capacity and pulmonary hemodynamics. PD5i are the recommended drugs for first-line PAH treatment, whereas sGCs are also the only drug approved for the treatment of resistant or inoperable chronic thromboembolic pulmonary hypertension. In this review, we will focus on the current information regarding the nitric oxide pathway and its modulation in PAH.
Keywords: nitric oxide; pulmonary arterial hypertension; right ventricle; riociguat.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Therapeutic augmentation of NO-sGC-cGMP signalling: lessons learned from pulmonary arterial hypertension and heart failure.Heart Fail Rev. 2022 Nov;27(6):1991-2003. doi: 10.1007/s10741-022-10239-5. Epub 2022 Apr 18. Heart Fail Rev. 2022. PMID: 35437713 Review.
-
A focus on riociguat in the treatment of pulmonary arterial hypertension.Basic Clin Pharmacol Toxicol. 2019 Sep;125(3):202-214. doi: 10.1111/bcpt.13272. Epub 2019 Jul 4. Basic Clin Pharmacol Toxicol. 2019. PMID: 31206240 Review.
-
[Optimization of specific therapy for pulmonary hypertension: the possibilities of riociguat].Ter Arkh. 2021 Sep 15;93(9):1117-1124. doi: 10.26442/00403660.2021.09.201014. Ter Arkh. 2021. PMID: 36286873 Russian.
-
Soluble guanylate cyclase stimulator riociguat and phosphodiesterase 5 inhibitor sildenafil ameliorate pulmonary hypertension due to left heart disease in mice.Int J Cardiol. 2016 Aug 1;216:85-91. doi: 10.1016/j.ijcard.2016.04.098. Epub 2016 Apr 16. Int J Cardiol. 2016. PMID: 27140341
-
Rationale and study design of RESPITE: An open-label, phase 3b study of riociguat in patients with pulmonary arterial hypertension who demonstrate an insufficient response to treatment with phosphodiesterase-5 inhibitors.Respir Med. 2017 Jan;122 Suppl 1:S18-S22. doi: 10.1016/j.rmed.2016.11.001. Epub 2016 Nov 5. Respir Med. 2017. PMID: 27887774 Clinical Trial.
Cited by
-
Long-Term Adverse Effects of Perinatal Hypoxia on the Adult Pulmonary Circulation Vary Between Males and Females in a Murine Model.Physiol Res. 2024 Nov 29;73(S2):S541-S556. doi: 10.33549/physiolres.935481. Physiol Res. 2024. PMID: 39589302 Free PMC article.
-
Circulating Intercellular Adhesion Molecule as a Novel Marker of Pulmonary Hypertension in Newborn.Indian Pediatr. 2025 Aug;62(8):605-607. doi: 10.1007/s13312-025-00037-1. Epub 2025 Apr 15. Indian Pediatr. 2025. PMID: 40232560
References
-
- Humbert M., Kovacs G., Hoeper M.M., Badagliacca R., Berger R.M.F., Brida M., Carlsen J., Coats A.J.S., Escribano-Subias P., Ferrari P., et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2023;62:2200879. doi: 10.1183/13993003.00879-2022. - DOI - PubMed
-
- Barst R.J., Rubin L.J., Long W.A., McGoon M.D., Rich S., Badesch D.B., Groves B.M., Tapson V.F., Bourge R.C., Brundage B.H., et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N. Engl. J. Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

