To Gain Insights into the Pathophysiological Mechanisms of the Thrombo-Inflammatory Process in the Atherosclerotic Plaque
- PMID: 38203218
- PMCID: PMC10778759
- DOI: 10.3390/ijms25010047
To Gain Insights into the Pathophysiological Mechanisms of the Thrombo-Inflammatory Process in the Atherosclerotic Plaque
Abstract
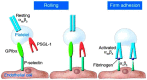
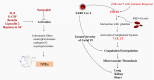
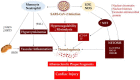
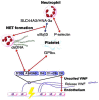
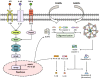
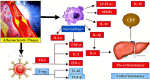
Thromboinflammation, the interplay between thrombosis and inflammation, is a significant pathway that drives cardiovascular and autoimmune diseases, as well as COVID-19. SARS-CoV-2 causes inflammation and blood clotting issues. Innate immune cells have emerged as key modulators of this process. Neutrophils, the most predominant white blood cells in humans, are strategically positioned to promote thromboinflammation. By releasing decondensed chromatin structures called neutrophil extracellular traps (NETs), neutrophils can initiate an organised cell death pathway. These structures are adorned with histones, cytoplasmic and granular proteins, and have cytotoxic, immunogenic, and prothrombotic effects that can hasten disease progression. Protein arginine deiminase 4 (PAD4) catalyses the citrullination of histones and is involved in the release of extracellular DNA (NETosis). The neutrophil inflammasome is also required for this process. Understanding the link between the immunological function of neutrophils and the procoagulant and proinflammatory activities of monocytes and platelets is important in understanding thromboinflammation. This text discusses how vascular blockages occur in thromboinflammation due to the interaction between neutrophil extracellular traps and ultra-large VWF (von Willebrand Factor). The activity of PAD4 is important for understanding the processes that drive thromboinflammation by linking the immunological function of neutrophils with the procoagulant and proinflammatory activities of monocytes and platelets. This article reviews how vaso-occlusive events in thrombo-inflammation occur through the interaction of neutrophil extracellular traps with von Willebrand factor. It highlights the relevance of PAD4 in neutrophil inflammasome assembly and neutrophil extracellular traps in thrombo-inflammatory diseases such as atherosclerosis and cardiovascular disease. Interaction between platelets, VWF, NETs and inflammasomes is critical for the progression of thromboinflammation in several diseases and was recently shown to be active in COVID-19.
Keywords: COVID-19; NETosis; neutrophil extracellular traps; neutrophil inflammasome; thromboinflammation; ultra-large VWF.
Conflict of interest statement
The author declares no conflict of interest.
Figures

Similar articles
-
Thromboinflammation: From Atherosclerosis to COVID-19.Arterioscler Thromb Vasc Biol. 2022 Sep;42(9):1103-1112. doi: 10.1161/ATVBAHA.122.317162. Epub 2022 Jul 8. Arterioscler Thromb Vasc Biol. 2022. PMID: 35861953 Free PMC article. Review.
-
Regulating Neutrophil PAD4/NOX-Dependent Cerebrovasular Thromboinflammation.Int J Biol Sci. 2023 Jan 9;19(3):852-864. doi: 10.7150/ijbs.77434. eCollection 2023. Int J Biol Sci. 2023. PMID: 36778112 Free PMC article.
-
PAD4 takes charge during neutrophil activation: Impact of PAD4 mediated NET formation on immune-mediated disease.J Thromb Haemost. 2021 Jul;19(7):1607-1617. doi: 10.1111/jth.15313. Epub 2021 May 12. J Thromb Haemost. 2021. PMID: 33773016 Free PMC article. Review.
-
Blood-brain crosstalk: the roles of neutrophils, platelets, and neutrophil extracellular traps in neuropathologies.Trends Neurosci. 2023 Sep;46(9):764-779. doi: 10.1016/j.tins.2023.06.005. Epub 2023 Jul 25. Trends Neurosci. 2023. PMID: 37500363 Review.
-
Targeting thromboinflammation in antiphospholipid syndrome.J Thromb Haemost. 2023 Apr;21(4):744-757. doi: 10.1016/j.jtha.2022.12.002. Epub 2022 Dec 22. J Thromb Haemost. 2023. PMID: 36696191 Review.
Cited by
-
The interplay of hydrogen sulfide and microRNAs in cardiovascular diseases: insights and future perspectives.Mamm Genome. 2024 Sep;35(3):309-323. doi: 10.1007/s00335-024-10043-6. Epub 2024 Jun 4. Mamm Genome. 2024. PMID: 38834923 Review.
-
Exploring catalase inhibition as an adjuvant to antimicrobial photodynamic therapy against Staphylococcus aureus.Photochem Photobiol Sci. 2025 Jun;24(6):1053-1068. doi: 10.1007/s43630-025-00735-6. Epub 2025 May 23. Photochem Photobiol Sci. 2025. PMID: 40410636
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

