Gene Characterization of Nocturnin Paralogues in Goldfish: Full Coding Sequences, Structure, Phylogeny and Tissue Expression
- PMID: 38203224
- PMCID: PMC10779419
- DOI: 10.3390/ijms25010054
Gene Characterization of Nocturnin Paralogues in Goldfish: Full Coding Sequences, Structure, Phylogeny and Tissue Expression
Abstract
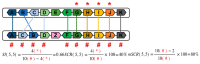
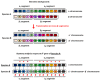
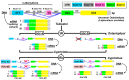
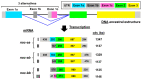
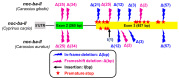
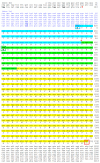
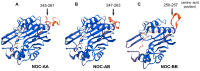
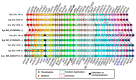
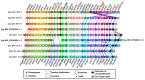
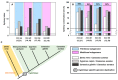
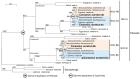
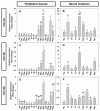
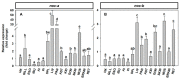
The aim of this work is the full characterization of all the nocturnin (noc) paralogues expressed in a teleost, the goldfish. An in silico analysis of the evolutive origin of noc in Osteichthyes is performed, including the splicing variants and new paralogues appearing after teleostean 3R genomic duplication and the cyprinine 4Rc. After sequencing the full-length mRNA of goldfish, we obtained two isoforms for noc-a (noc-aa and noc-ab) with two splice variants (I and II), and only one for noc-b (noc-bb) with two transcripts (II and III). Using the splicing variant II, the prediction of the secondary and tertiary structures renders a well-conserved 3D distribution of four α-helices and nine β-sheets in the three noc isoforms. A synteny analysis based on the localization of noc genes in the patrilineal or matrilineal subgenomes and a phylogenetic tree of protein sequences were accomplished to stablish a classification and a long-lasting nomenclature of noc in goldfish, and valid to be extrapolated to allotetraploid Cyprininae. Finally, both goldfish and zebrafish showed a broad tissue expression of all the noc paralogues. Moreover, the enriched expression of specific paralogues in some tissues argues in favour of neo- or subfunctionalization.
Keywords: Carassius auratus; gene evolution; molecular cloning; nocturnin nomenclature; phylogenetic tree; protein structure; synteny analysis; synteny quantification; tissue distribution; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
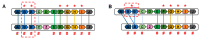




Similar articles
-
First evidence of nocturnin in fish: two isoforms in goldfish differentially regulated by feeding.Am J Physiol Regul Integr Comp Physiol. 2018 Feb 1;314(2):R304-R312. doi: 10.1152/ajpregu.00241.2017. Epub 2017 Nov 14. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29070504
-
Molecular characterization of novel interferon gamma receptor 1 isoforms in zebrafish (Danio rerio) and goldfish (Carassius auratus L.).Mol Immunol. 2009 Sep;46(15):3050-9. doi: 10.1016/j.molimm.2009.06.004. Epub 2009 Jul 3. Mol Immunol. 2009. PMID: 19577303
-
Evolutionary history of c-myc in teleosts and characterization of the duplicated c-myca genes in goldfish embryos.Mol Reprod Dev. 2012 Feb;79(2):85-96. doi: 10.1002/mrd.22004. Epub 2011 Dec 28. Mol Reprod Dev. 2012. PMID: 22213278
-
Identification and molecular characterization of the interleukin-10 receptor 1 of the zebrafish (Danio rerio) and the goldfish (Carassius auratus L.).Dev Comp Immunol. 2012 Feb;36(2):408-17. doi: 10.1016/j.dci.2011.08.006. Epub 2011 Aug 26. Dev Comp Immunol. 2012. PMID: 21906622
-
Differential tissue distribution, developmental programming, estrogen regulation and promoter characteristics of cyp19 genes in teleost fish.J Steroid Biochem Mol Biol. 2001 Dec;79(1-5):305-14. doi: 10.1016/s0960-0760(01)00147-9. J Steroid Biochem Mol Biol. 2001. PMID: 11850237 Review.
Cited by
-
Zebrafish navigating the metabolic maze: insights into human disease - assets, challenges and future implications.J Diabetes Metab Disord. 2024 Dec 16;24(1):3. doi: 10.1007/s40200-024-01539-8. eCollection 2025 Jun. J Diabetes Metab Disord. 2024. PMID: 39697864 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

