Long Non-Coding RNAs: Bridging Cancer-Associated Thrombosis and Clinical Outcome of Ovarian Cancer Patients
- PMID: 38203310
- PMCID: PMC10778953
- DOI: 10.3390/ijms25010140
Long Non-Coding RNAs: Bridging Cancer-Associated Thrombosis and Clinical Outcome of Ovarian Cancer Patients
Abstract
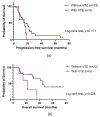
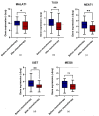
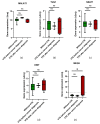
Ovarian cancer (OC) and venous thromboembolism (VTE) have a close relationship, in which tumour cells surpass the haemostatic system to drive cancer progression. Long non-coding RNAs (lncRNAs) have been implicated in VTE pathogenesis, yet their roles in cancer-associated thrombosis (CAT) and their prognostic value are unexplored. Understanding how these lncRNAs influence venous thrombogenesis and ovarian tumorigenesis may lead to the identification of valuable biomarkers for VTE and OC management. Thus, this study evaluated the impact of five lncRNAs, namely MALAT1, TUG1, NEAT1, XIST and MEG8, on a cohort of 40 OC patients. Patients who developed VTE after OC diagnosis had worse overall survival compared to their counterparts (log-rank test, p = 0.028). Elevated pre-chemotherapy MEG8 levels in peripheral blood cells (PBCs) predicted VTE after OC diagnosis (Mann-Whitney U test, p = 0.037; Χ2 test, p = 0.033). In opposition, its low levels were linked to a higher risk of OC progression (adjusted hazard ratio (aHR) = 3.00; p = 0.039). Furthermore, low pre-chemotherapy NEAT1 levels in PBCs were associated with a higher risk of death (aHR = 6.25; p = 0.008). As for the remaining lncRNAs, no significant association with VTE incidence, OC progression or related mortality was observed. Future investigation with external validation in larger cohorts is needed to dissect the implications of the evaluated lncRNAs in OC patients.
Keywords: RNA; epithelial ovarian carcinoma; long non-coding; prognosis; thromboprophylaxis; venous thromboembolism.
Conflict of interest statement
J.L.-P. has received a research Grant from GESCAT-Grupo de Estudos de Cancro e Trombose. This institution had no role in the decision to conduct the study, write and publish this manuscript. The remaining authors declare no conflict of interest.
Figures
Similar articles
-
Plasma microRNA Environment Linked to Tissue Factor Pathway and Cancer-Associated Thrombosis: Prognostic Significance in Ovarian Cancer.Biomolecules. 2024 Jul 31;14(8):928. doi: 10.3390/biom14080928. Biomolecules. 2024. PMID: 39199316 Free PMC article.
-
Haemostatic Gene Expression in Cancer-Related Immunothrombosis: Contribution for Venous Thromboembolism and Ovarian Tumour Behaviour.Cancers (Basel). 2024 Jun 27;16(13):2356. doi: 10.3390/cancers16132356. Cancers (Basel). 2024. PMID: 39001418 Free PMC article.
-
Heritable Genetic Variability in Ovarian Tumours: Exploring Venous Thromboembolism Susceptibility and Cancer Prognosis in a Hospital-Based Study.Gene. 2025 May 20;950:149378. doi: 10.1016/j.gene.2025.149378. Epub 2025 Mar 1. Gene. 2025. PMID: 40032058
-
The prevalence, risk factors, and prognostic value of venous thromboembolism in ovarian cancer patients receiving chemotherapy: a systematic review and meta-analysis.World J Surg Oncol. 2021 Jan 13;19(1):12. doi: 10.1186/s12957-020-02101-5. World J Surg Oncol. 2021. PMID: 33441137 Free PMC article.
-
Long Non-Coding RNAs in Venous Thromboembolism: Where Do We Stand?Int J Mol Sci. 2023 Jul 28;24(15):12103. doi: 10.3390/ijms241512103. Int J Mol Sci. 2023. PMID: 37569483 Free PMC article. Review.
Cited by
-
Plasma microRNA Environment Linked to Tissue Factor Pathway and Cancer-Associated Thrombosis: Prognostic Significance in Ovarian Cancer.Biomolecules. 2024 Jul 31;14(8):928. doi: 10.3390/biom14080928. Biomolecules. 2024. PMID: 39199316 Free PMC article.
-
Endothelial Dysfunction Markers in Ovarian Cancer: VTE Risk and Tumour Prognostic Outcomes.Life (Basel). 2024 Dec 9;14(12):1630. doi: 10.3390/life14121630. Life (Basel). 2024. PMID: 39768338 Free PMC article.
-
Haemostatic Gene Expression in Cancer-Related Immunothrombosis: Contribution for Venous Thromboembolism and Ovarian Tumour Behaviour.Cancers (Basel). 2024 Jun 27;16(13):2356. doi: 10.3390/cancers16132356. Cancers (Basel). 2024. PMID: 39001418 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

