Genetic Alterations of NF-κB and Its Regulators: A Rich Platform to Advance Colorectal Cancer Diagnosis and Treatment
- PMID: 38203325
- PMCID: PMC10779007
- DOI: 10.3390/ijms25010154
Genetic Alterations of NF-κB and Its Regulators: A Rich Platform to Advance Colorectal Cancer Diagnosis and Treatment
Abstract
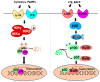
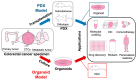
Colorectal cancer (CRC) is the third leading cause of cancer mortality in the United States, with an estimated 52,000 deaths in 2023. Though significant progress has been made in both diagnosis and treatment of CRC in recent years, genetic heterogeneity of CRC-the culprit for possible CRC relapse and drug resistance, is still an insurmountable challenge. Thus, developing more effective therapeutics to overcome this challenge in new CRC treatment strategies is imperative. Genetic and epigenetic changes are well recognized to be responsible for the stepwise development of CRC malignancy. In this review, we focus on detailed genetic alteration information about the nuclear factor (NF)-κB signaling, including both NF-κB family members, and their regulators, such as protein arginine methyltransferase 5 (PRMT5), and outer dynein arm docking complex subunit 2 (ODAD2, also named armadillo repeat-containing 4, ARMC4), etc., in CRC patients. Moreover, we provide deep insight into different CRC research models, with a particular focus on patient-derived xenografts (PDX) and organoid models, and their potential applications in CRC research. Genetic alterations on NF-κB signaling components are estimated to be more than 50% of the overall genetic changes identified in CRC patients collected by cBioportal for Cancer Genomics; thus, emphasizing its paramount importance in CRC progression. Consequently, various genetic alterations on NF-κB signaling may hold great promise for novel therapeutic development in CRC. Future endeavors may focus on utilizing CRC models (e.g., PDX or organoids, or isogenic human embryonic stem cell (hESC)-derived colonic cells, or human pluripotent stem cells (hPSC)-derived colonic organoids, etc.) to further uncover the underpinning mechanism of these genetic alterations in NF-κB signaling in CRC progression. Moreover, establishing platforms for drug discovery in dishes, and developing Biobanks, etc., may further pave the way for the development of innovative personalized medicine to treat CRC in the future.
Keywords: CRC; NF-κB; ODAD2; PDX; PRMT5; genetic alteration; organoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Using VBIM Technique to Discover ARMC4/ODAD2 as a Novel Negative Regulator of NF-κB and a New Tumor Suppressor in Colorectal Cancer.Int J Mol Sci. 2022 Mar 1;23(5):2732. doi: 10.3390/ijms23052732. Int J Mol Sci. 2022. PMID: 35269880 Free PMC article.
-
PRMT5-mediated methylation of YBX1 regulates NF-κB activity in colorectal cancer.Sci Rep. 2020 Sep 28;10(1):15934. doi: 10.1038/s41598-020-72942-3. Sci Rep. 2020. PMID: 32985589 Free PMC article.
-
The Pivotal Player: Components of NF-κB Pathway as Promising Biomarkers in Colorectal Cancer.Int J Mol Sci. 2021 Jul 11;22(14):7429. doi: 10.3390/ijms22147429. Int J Mol Sci. 2021. PMID: 34299049 Free PMC article. Review.
-
Regulation of a PRMT5/NF-κB Axis by Phosphorylation of PRMT5 at Serine 15 in Colorectal Cancer.Int J Mol Sci. 2020 May 23;21(10):3684. doi: 10.3390/ijms21103684. Int J Mol Sci. 2020. PMID: 32456215 Free PMC article.
-
Protein arginine N-methyltransferase 5 in colorectal carcinoma: Insights into mechanisms of pathogenesis and therapeutic strategies.Biomed Pharmacother. 2022 Jan;145:112368. doi: 10.1016/j.biopha.2021.112368. Epub 2021 Nov 15. Biomed Pharmacother. 2022. PMID: 34794114 Review.
Cited by
-
NF-κB pathway and angiogenesis: insights into colorectal cancer development and therapeutic targets.Eur J Med Res. 2024 Dec 19;29(1):610. doi: 10.1186/s40001-024-02168-w. Eur J Med Res. 2024. PMID: 39702532 Free PMC article. Review.
-
Study on the effect and mechanism of Lacticaseibacillus rhamnosus AFY06 on inflammation-associated colorectal cancer induced by AOM/DSS in mice.Front Microbiol. 2024 Mar 20;15:1382781. doi: 10.3389/fmicb.2024.1382781. eCollection 2024. Front Microbiol. 2024. PMID: 38572238 Free PMC article.
-
Anticancer Activity of Roburic Acid: In Vitro and In Silico Investigation.Int J Mol Sci. 2025 Jul 3;26(13):6420. doi: 10.3390/ijms26136420. Int J Mol Sci. 2025. PMID: 40650205 Free PMC article.
-
Regulatory role of PPAR in colorectal cancer.Cell Death Discov. 2025 Jan 28;11(1):28. doi: 10.1038/s41420-025-02313-2. Cell Death Discov. 2025. PMID: 39875357 Free PMC article. Review.
-
Closing Editorial: Colorectal Cancer-A Molecular Genetics Perspective.Int J Mol Sci. 2024 Nov 24;25(23):12604. doi: 10.3390/ijms252312604. Int J Mol Sci. 2024. PMID: 39684316 Free PMC article.
References
-
- Hossain M.S., Karuniawati H., Jairoun A.A., Urbi Z., Ooi D.J., John A., Lim Y.C., Kibria K.M.K., Mohiuddin A.K.M., Ming L.C., et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers. 2022;14:1732. doi: 10.3390/cancers14071732. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

