A Cancer-Specific Monoclonal Antibody against Podocalyxin Exerted Antitumor Activities in Pancreatic Cancer Xenografts
- PMID: 38203331
- PMCID: PMC10779310
- DOI: 10.3390/ijms25010161
A Cancer-Specific Monoclonal Antibody against Podocalyxin Exerted Antitumor Activities in Pancreatic Cancer Xenografts
Abstract
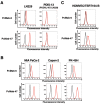
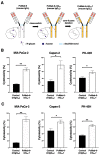
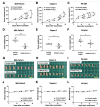
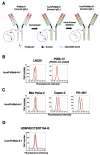
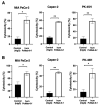
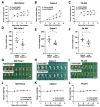
Podocalyxin (PODXL) overexpression is associated with poor clinical outcomes in various tumors. PODXL is involved in tumor malignant progression through the promotion of invasiveness and metastasis. Therefore, PODXL is considered a promising target of monoclonal antibody (mAb)-based therapy. However, PODXL also plays an essential role in normal cells, such as vascular and lymphatic endothelial cells. Therefore, cancer specificity or selectivity is required to reduce adverse effects on normal cells. Here, we developed an anti-PODXL cancer-specific mAb (CasMab), PcMab-6 (IgG1, kappa), by immunizing mice with a soluble PODXL ectodomain derived from a glioblastoma LN229 cell. PcMab-6 reacted with the PODXL-positive LN229 cells but not with PODXL-knockout LN229 cells in flow cytometry. Importantly, PcMab-6 recognized pancreatic ductal adenocarcinoma (PDAC) cell lines (MIA PaCa-2, Capan-2, and PK-45H) but did not react with normal lymphatic endothelial cells (LECs). In contrast, one of the non-CasMabs, PcMab-47, showed high reactivity to both the PDAC cell lines and LECs. Next, we engineered PcMab-6 into a mouse IgG2a-type (PcMab-6-mG2a) and a humanized IgG1-type (humPcMab-6) mAb and further produced the core fucose-deficient types (PcMab-6-mG2a-f and humPcMab-6-f, respectively) to potentiate the antibody-dependent cellular cytotoxicity (ADCC). Both PcMab-6-mG2a-f and humPcMab-6-f exerted ADCC and complement-dependent cellular cytotoxicity in the presence of effector cells and complements, respectively. In the PDAC xenograft model, both PcMab-6-mG2a-f and humPcMab-6-f exhibited potent antitumor effects. These results indicated that humPcMab-6-f could apply to antibody-based therapy against PODXL-expressing pancreatic cancers.
Keywords: PODXL; cancer-specific monoclonal antibody; defucosylated antibody; pancreatic cancer; podocalyxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Choo A.B., Tan H.L., Ang S.N., Fong W.J., Chin A., Lo J., Zheng L., Hentze H., Philp R.J., Oh S.K., et al. Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells. 2008;26:1454–1463. doi: 10.1634/stemcells.2007-0576. - DOI - PubMed
-
- Kriehuber E., Breiteneder-Geleff S., Groeger M., Soleiman A., Schoppmann S.F., Stingl G., Kerjaschki D., Maurer D. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

