Evaluation of Immune Response to Mucosal Immunization with an Oral Probiotic-Based Vaccine in Mice: Potential for Prime-Boost Immunization against SARS-CoV-2
- PMID: 38203387
- PMCID: PMC10779021
- DOI: 10.3390/ijms25010215
Evaluation of Immune Response to Mucosal Immunization with an Oral Probiotic-Based Vaccine in Mice: Potential for Prime-Boost Immunization against SARS-CoV-2
Abstract
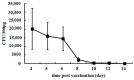
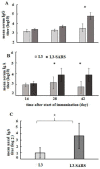
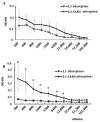
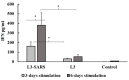
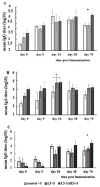
Following the conclusion of the COVID-19 pandemic, the persistent genetic variability in the virus and its ongoing circulation within the global population necessitate the enhancement of existing preventive vaccines and the development of novel ones. A while back, we engineered an orally administered probiotic-based vaccine, L3-SARS, by integrating a gene fragment that encodes the spike protein S of the SARS-CoV-2 virus into the genome of the probiotic strain E. faecium L3, inducing the expression of viral antigen on the surface of bacteria. Previous studies demonstrated the efficacy of this vaccine candidate in providing protection against the virus in Syrian hamsters. In this present study, utilizing laboratory mice, we assess the immune response subsequent to immunization via the gastrointestinal mucosa and discuss its potential as an initial phase in a two-stage vaccination strategy. Our findings indicate that the oral administration of L3-SARS elicits an adaptive immune response in mice. Pre-immunization with L3-SARS enhances and prolongs the humoral immune response following a single subcutaneous immunization with a recombinant S-protein analogous to the S-insert of the coronavirus in Enterococcus faecium L3.
Keywords: Enterococcus faecium L3; S protein; SARS-CoV-2; immune response; mucosal vaccines; prime-boost immunization; probiotic-based vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
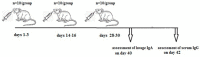

Similar articles
-
Intranasal administration of unadjuvanted SARS-CoV-2 spike antigen boosts antigen-specific immune responses induced by parenteral protein subunit vaccine prime in mice and hamsters.Eur J Immunol. 2024 Jun;54(6):e2350620. doi: 10.1002/eji.202350620. Epub 2024 Apr 1. Eur J Immunol. 2024. PMID: 38561974
-
An Intranasal OMV-Based Vaccine Induces High Mucosal and Systemic Protecting Immunity Against a SARS-CoV-2 Infection.Front Immunol. 2021 Dec 17;12:781280. doi: 10.3389/fimmu.2021.781280. eCollection 2021. Front Immunol. 2021. PMID: 34987509 Free PMC article.
-
Short- and Long-Interval Prime-Boost Vaccination with the Candidate Vaccines MVA-SARS-2-ST and MVA-SARS-2-S Induces Comparable Humoral and Cell-Mediated Immunity in Mice.Viruses. 2023 May 17;15(5):1180. doi: 10.3390/v15051180. Viruses. 2023. PMID: 37243266 Free PMC article.
-
Intranasal immunization with the recombinant measles virus encoding the spike protein of SARS-CoV-2 confers protective immunity against COVID-19 in hamsters.Vaccine. 2024 Jan 12;42(2):69-74. doi: 10.1016/j.vaccine.2023.12.011. Epub 2023 Dec 14. Vaccine. 2024. PMID: 38097457
-
Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects.Rev Med Virol. 2021 May;31(3):e2183. doi: 10.1002/rmv.2183. Epub 2020 Oct 15. Rev Med Virol. 2021. PMID: 33594794 Free PMC article. Review.
References
-
- Possas C., de Souza Antunes A.M., de Oliveira A.M., de Souza Mendes Santos C.D., Ramos M.P., de Oliveira Rodrigues Schumacher S., Homma A. Vaccine Innovation for Pandemic Preparedness: Patent Landscape, Global Sustainability, and Circular Bioeconomy in Post-COVID-19 era. Circ. Econ. Sustain. 2021;1:1439–1461. doi: 10.1007/s43615-021-00051-y. - DOI - PMC - PubMed
-
- Soleimanpour S., Yaghoubi A. COVID-19 vaccine: Where are we now and where should we go? Infect. Dis. Immun. 2021;20:43–51. doi: 10.1080/14760584.2021.1875824. - DOI
-
- Li T., Zhang T., Gu Y., Li S., Xia N. Current progress and challenges in the design and development of a successful COVID-19 vaccine. Fundam. Res. 2021;1:139–150. doi: 10.1016/j.fmre.2021.01.011. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

