Elevated Expression of HSP72 in the Prefrontal Cortex and Hippocampus of Rats Subjected to Chronic Mild Stress and Treated with Imipramine
- PMID: 38203414
- PMCID: PMC10779295
- DOI: 10.3390/ijms25010243
Elevated Expression of HSP72 in the Prefrontal Cortex and Hippocampus of Rats Subjected to Chronic Mild Stress and Treated with Imipramine
Abstract
The HSP70 and HSP90 family members belong to molecular chaperones that exhibit protective functions during the cellular response to stressful agents. We investigated whether the exposure of rats to chronic mild stress (CMS), a validated model of depression, affects the expression of HSP70 and HSP90 in the prefrontal cortex (PFC), hippocampus (HIP) and thalamus (Thal). Male Wistar rats were exposed to CMS for 3 or 8 weeks. The antidepressant imipramine (IMI, 10 mg/kg, i.p., daily) was introduced in the last five weeks of the long-term CMS procedure. Depressive-like behavior was verified by the sucrose consumption test. The expression of mRNA and protein was quantified by real-time PCR and Western blot, respectively. In the 8-week CMS model, stress alone elevated HSP72 and HSP90B mRNA expression in the HIP. HSP72 mRNA was increased in the PFC and HIP of rats not responding to IMI treatment vs. IMI responders. The CMS exposure increased HSP72 protein expression in the cytosolic fraction of the PFC and HIP, and this effect was diminished by IMI treatment. Our results suggest that elevated levels of HSP72 may serve as an important indicator of neuronal stress reactions accompanying depression pathology and could be a potential target for antidepressant strategy.
Keywords: HSP70; HSP90; chronic mild stress; constitutive HSC70; constitutive HSP90B; depression; heat shock protein; imipramine; inducible HSP72; inducible HSP90A.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

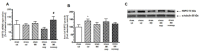
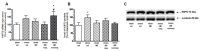
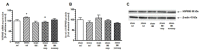
Similar articles
-
Interaction of the immune-inflammatory and the kynurenine pathways in rats resistant to antidepressant treatment in model of depression.Int Immunopharmacol. 2019 Aug;73:527-538. doi: 10.1016/j.intimp.2019.05.039. Epub 2019 Jun 5. Int Immunopharmacol. 2019. PMID: 31176083
-
Chronic mild stress and imipramine treatment elicit opposite changes in behavior and in gene expression in the mouse prefrontal cortex.Pharmacol Biochem Behav. 2015 Aug;135:227-36. doi: 10.1016/j.pbb.2015.06.001. Epub 2015 Jun 4. Pharmacol Biochem Behav. 2015. PMID: 26051025
-
The Effect of Chronic Mild Stress and Imipramine on the Markers of Oxidative Stress and Antioxidant System in Rat Liver.Neurotox Res. 2016 Aug;30(2):173-84. doi: 10.1007/s12640-016-9614-8. Epub 2016 Mar 10. Neurotox Res. 2016. PMID: 26961706 Free PMC article.
-
Antidepressant administration modulates stress-induced DNA methylation and DNA methyltransferase expression in rat prefrontal cortex and hippocampus.Behav Brain Res. 2018 May 2;343:8-15. doi: 10.1016/j.bbr.2018.01.022. Epub 2018 Jan 31. Behav Brain Res. 2018. PMID: 29378290
-
Repeated co-treatment with imipramine and amantadine induces hippocampal brain-derived neurotrophic factor gene expression in rats.J Physiol Pharmacol. 2007 Jun;58(2):219-34. J Physiol Pharmacol. 2007. PMID: 17622693
Cited by
-
Restraint stress effects on glutamate signaling protein levels in the rats' frontal cortex: Does β1 adrenoceptor activity matter?Front Pharmacol. 2025 Jan 6;15:1451895. doi: 10.3389/fphar.2024.1451895. eCollection 2024. Front Pharmacol. 2025. PMID: 39834820 Free PMC article.
-
Psychobiotics in Depression: Sources, Metabolites, and Treatment-A Systematic Review.Nutrients. 2025 Jun 27;17(13):2139. doi: 10.3390/nu17132139. Nutrients. 2025. PMID: 40647242 Free PMC article. Review.
-
Pipette-tip solid-phase extraction coupled with matrix-assisted laser desorption/ionization mass spectrometry enables rapid and high-throughput analysis of antidepressants in rat serum.Anal Bioanal Chem. 2024 Sep;416(22):5013-5023. doi: 10.1007/s00216-024-05439-x. Epub 2024 Jul 13. Anal Bioanal Chem. 2024. PMID: 38997460
-
Trends in research on novel antidepressant treatments.Front Pharmacol. 2025 Jan 27;16:1544795. doi: 10.3389/fphar.2025.1544795. eCollection 2025. Front Pharmacol. 2025. PMID: 39931695 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

