Developmental and Nutritional Dynamics of Malpighian Tubule Autofluorescence in the Asian Tiger Mosquito Aedes albopictus
- PMID: 38203417
- PMCID: PMC10778832
- DOI: 10.3390/ijms25010245
Developmental and Nutritional Dynamics of Malpighian Tubule Autofluorescence in the Asian Tiger Mosquito Aedes albopictus
Abstract
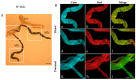
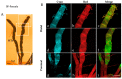
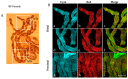
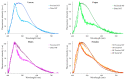
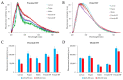
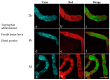
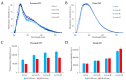
Malpighian tubules (MTs) are arthropod excretory organs crucial for the osmoregulation, detoxification and excretion of xenobiotics and metabolic wastes, which include tryptophan degradation products along the kynurenine (KYN) pathway. Specifically, the toxic intermediate 3-hydroxy kynurenine (3-HK) is metabolized through transamination to xanthurenic acid or in the synthesis of ommochrome pigments. Early investigations in Drosophila larval fat bodies revealed an intracellular autofluorescence (AF) that depended on tryptophan administration. Subsequent observations documented AF changes in the MTs of Drosophila eye-color mutants genetically affecting the conversion of tryptophan to KYN or 3-HK and the intracellular availability of zinc ions. In the present study, the AF properties of the MTs in the Asian tiger mosquito, Aedes albopictus, were characterized in different stages of the insect's life cycle, tryptophan-administered larvae and blood-fed adult females. Confocal imaging and microspectroscopy showed AF changes in the distribution of intracellular, brilliant granules and in the emission spectral shape and amplitude between the proximal and distal segments of MTs across the different samples. The findings suggest AF can serve as a promising marker for investigating the functional status of MTs in response to metabolic alterations, contributing to the use of MTs as a potential research model in biomedicine.
Keywords: confocal microscopy; dietary intake; emission spectra; fluorescent granules; mosquito developmental stages; principal cells; spectrofluorimetric analysis; tryptophan.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses or interpretation of the data, in the writing of the manuscript or in the decision to publish the results.
Figures



Similar articles
-
Effect of tryptophan metabolites on fluorescent granules in the Malpighian tubules of eye color mutants of Drosophila melanogaster.Zoolog Sci. 1996 Feb;13(1):97-104. doi: 10.2108/zsj.13.97. Zoolog Sci. 1996. PMID: 8688814
-
Transcriptomic evidence for a dramatic functional transition of the malpighian tubules after a blood meal in the Asian tiger mosquito Aedes albopictus.PLoS Negl Trop Dis. 2014 Jun 5;8(6):e2929. doi: 10.1371/journal.pntd.0002929. eCollection 2014 Jun. PLoS Negl Trop Dis. 2014. PMID: 24901705 Free PMC article.
-
Malpighian Tubules as Novel Targets for Mosquito Control.Int J Environ Res Public Health. 2017 Jan 24;14(2):111. doi: 10.3390/ijerph14020111. Int J Environ Res Public Health. 2017. PMID: 28125032 Free PMC article. Review.
-
Effect of Kynurenic Acid on Pupae Viability of Drosophila melanogaster cinnabar and cardinal Eye Color Mutants with Altered Tryptophan-Kynurenine Metabolism.Neurotox Res. 2018 Aug;34(2):324-331. doi: 10.1007/s12640-018-9891-5. Epub 2018 Apr 4. Neurotox Res. 2018. PMID: 29619629
-
Hormonal regulation and functional role of the "renal" tubules in the disease vector, Aedes aegypti.Vitam Horm. 2021;117:189-225. doi: 10.1016/bs.vh.2021.06.007. Epub 2021 Aug 9. Vitam Horm. 2021. PMID: 34420581 Review.
Cited by
-
In situ detection of ferric reductase activity in the intestinal lumen of an insect.J Biol Inorg Chem. 2024 Dec;29(7-8):773-784. doi: 10.1007/s00775-024-02080-y. Epub 2024 Dec 1. J Biol Inorg Chem. 2024. PMID: 39617837 Free PMC article.
References
-
- Piermarini P.M., Gillen C.M. Non-traditional models: The molecular physiology of sodium and water transport in mosquito Malpighian tubules. In: Hyndman K., Pannabecker T., editors. Sodium and Water Homeostasis. Springer; New York, NY, USA: 2015. pp. 255–278.
-
- Xu J., Liu Y., Li H., Tarashansky A.J., Kalicki C.H., Hung R.J., Hu Y., Comjean A., Kolluru S.S., Wang B., et al. Transcriptional and functional motifs defining renal function revealed by single-nucleus RNA sequencing. Proc. Natl. Acad. Sci. USA. 2022;119:e2203179119. doi: 10.1073/pnas.2203179119. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

