Re-Arranging the Puzzle between the Amyloid-Beta and Tau Pathology: An APP-Centric Approach
- PMID: 38203429
- PMCID: PMC10779219
- DOI: 10.3390/ijms25010259
Re-Arranging the Puzzle between the Amyloid-Beta and Tau Pathology: An APP-Centric Approach
Abstract
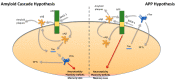
After several years of research in the field of Alzheimer's disease (AD), it is still unclear how amyloid-beta (Aβ) and Tau, two key hallmarks of the disease, mediate the neuropathogenic events that lead to AD. Current data challenge the "Amyloid Cascade Hypothesis" that has prevailed in the field of AD, stating that Aβ precedes and triggers Tau pathology that will eventually become the toxic entity in the progression of the disease. This perspective also led the field of therapeutic approaches towards the development of strategies that target Aβ or Tau. In the present review, we discuss recent literature regarding the neurotoxic role of both Aβ and Tau in AD, as well as their physiological function in the healthy brain. Consequently, we present studies suggesting that Aβ and Tau act independently of each other in mediating neurotoxicity in AD, thereafter, re-evaluating the "Amyloid Cascade Hypothesis" that places Tau pathology downstream of Aβ. More recent studies have confirmed that both Aβ and Tau could propagate the disease and induce synaptic and memory impairments via the amyloid precursor protein (APP). This finding is not only interesting from a mechanistic point of view since it provides better insights into the AD pathogenesis but also from a therapeutic point of view since it renders APP a common downstream effector for both Aβ and Tau. Subsequently, therapeutic strategies that act on APP might provide a more viable and physiologically relevant approach for targeting AD.
Keywords: Tau; amyloid cascade hypothesis; amyloid-beta; amyloid-precursor protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Role of Amyloid-β and Tau Proteins in Alzheimer's Disease: Confuting the Amyloid Cascade.J Alzheimers Dis. 2018;64(s1):S611-S631. doi: 10.3233/JAD-179935. J Alzheimers Dis. 2018. PMID: 29865055 Free PMC article. Review.
-
Key Peptides and Proteins in Alzheimer's Disease.Curr Protein Pept Sci. 2019;20(6):577-599. doi: 10.2174/1389203720666190103123434. Curr Protein Pept Sci. 2019. PMID: 30605056 Review.
-
Metformin attenuates plaque-associated tau pathology and reduces amyloid-β burden in APP/PS1 mice.Alzheimers Res Ther. 2021 Feb 9;13(1):40. doi: 10.1186/s13195-020-00761-9. Alzheimers Res Ther. 2021. PMID: 33563332 Free PMC article.
-
Synaptic Mitochondria: An Early Target of Amyloid-β and Tau in Alzheimer's Disease.J Alzheimers Dis. 2021;84(4):1391-1414. doi: 10.3233/JAD-215139. J Alzheimers Dis. 2021. PMID: 34719499 Review.
Cited by
-
Advancements in the investigation of the mechanisms underlying cognitive aging.Biogerontology. 2025 Aug 10;26(4):158. doi: 10.1007/s10522-025-10300-4. Biogerontology. 2025. PMID: 40783909 Free PMC article. Review.
-
Advances in gait research related to Alzheimer's disease.Front Neurol. 2025 Jun 3;16:1548283. doi: 10.3389/fneur.2025.1548283. eCollection 2025. Front Neurol. 2025. PMID: 40529444 Free PMC article.
-
Integrative approaches in Alzheimer's disease: evaluating the potential of traditional, complementary, and integrative medicine (TCIM).Front Pharmacol. 2025 Jun 30;16:1561702. doi: 10.3389/fphar.2025.1561702. eCollection 2025. Front Pharmacol. 2025. PMID: 40661073 Free PMC article. Review.
-
Investigating the Effect and Mechanism of 3-Methyladenine Against Diabetic Encephalopathy by Network Pharmacology, Molecular Docking, and Experimental Validation.Pharmaceuticals (Basel). 2025 Apr 22;18(5):605. doi: 10.3390/ph18050605. Pharmaceuticals (Basel). 2025. PMID: 40430426 Free PMC article.
References
-
- Shinohara M., Sato N., Shimamura M., Kurinami H., Hamasaki T., Chatterjee A., Rakugi H., Morishita R. Possible modification of Alzheimer’s disease by statins in midlife: Interactions with genetic and non-genetic risk factors. Front. Aging Neurosci. 2014;6:71. doi: 10.3389/fnagi.2014.00071. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

