The Potential of Congo Red Supplied Aggregates of Multitargeted Tyrosine Kinase Inhibitor (Sorafenib, BAY-43-9006) in Enhancing Therapeutic Impact on Bladder Cancer
- PMID: 38203437
- PMCID: PMC10779242
- DOI: 10.3390/ijms25010269
The Potential of Congo Red Supplied Aggregates of Multitargeted Tyrosine Kinase Inhibitor (Sorafenib, BAY-43-9006) in Enhancing Therapeutic Impact on Bladder Cancer
Abstract
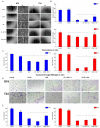
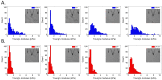
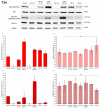
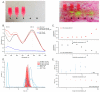
Bladder cancer is a common malignancy associated with high recurrence rates and potential progression to invasive forms. Sorafenib, a multi-targeted tyrosine kinase inhibitor, has shown promise in anti-cancer therapy, but its cytotoxicity to normal cells and aggregation in solution limits its clinical application. To address these challenges, we investigated the formation of supramolecular aggregates of sorafenib with Congo red (CR), a bis-azo dye known for its supramolecular interaction. We analyzed different mole ratios of CR-sorafenib aggregates and evaluated their effects on bladder cancer cells of varying levels of malignancy. In addition, we also evaluated the effect of the test compounds on normal uroepithelial cells. Our results demonstrated that sorafenib inhibits the proliferation of bladder cancer cells and induces apoptosis in a dose-dependent manner. However, high concentrations of sorafenib also showed cytotoxicity to normal uroepithelial cells. In contrast, the CR-BAY aggregates exhibited reduced cytotoxicity to normal cells while maintaining anti-cancer activity. The aggregates inhibited cancer cell migration and invasion, suggesting their potential for metastasis prevention. Dynamic light scattering and UV-VIS measurements confirmed the formation of stable co-aggregates with distinctive spectral properties. These CR-sorafenib aggregates may provide a promising approach to targeted therapy with reduced cytotoxicity and improved stability for drug delivery in bladder cancer treatment. This work shows that the drug-excipient aggregates proposed and described so far, as Congo red-sorafenib, can be a real step forward in anti-cancer therapies.
Keywords: Congo red; bladder cancer; multidisciplinary treatment; personalized medicine; tyrosine kinases inhibitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Interaction of Avapritinib with Congo Red in Pancreatic Cancer Cells: Molecular Modeling and Biophysical Studies.Int J Mol Sci. 2025 Feb 25;26(5):1980. doi: 10.3390/ijms26051980. Int J Mol Sci. 2025. PMID: 40076604 Free PMC article.
-
Stimulatory effects of the multi-kinase inhibitor sorafenib on human bladder cancer cells.Br J Pharmacol. 2010 Aug;160(7):1690-8. doi: 10.1111/j.1476-5381.2010.00838.x. Br J Pharmacol. 2010. PMID: 20649572 Free PMC article.
-
Antibacterial Therapy by Ag+ Ions Complexed with Titan Yellow/Congo Red and Albumin during Anticancer Therapy of Urinary Bladder Cancer.Int J Mol Sci. 2021 Dec 21;23(1):26. doi: 10.3390/ijms23010026. Int J Mol Sci. 2021. PMID: 35008445 Free PMC article.
-
Multiple mechanisms mediate resistance to sorafenib in urothelial cancer.Int J Mol Sci. 2014 Nov 7;15(11):20500-17. doi: 10.3390/ijms151120500. Int J Mol Sci. 2014. PMID: 25387078 Free PMC article.
-
Sorafenib (BAY 43-9006): review of clinical development.Curr Clin Pharmacol. 2006 Sep;1(3):223-8. doi: 10.2174/157488406778249325. Curr Clin Pharmacol. 2006. PMID: 18666747 Review.
Cited by
-
Interaction of Avapritinib with Congo Red in Pancreatic Cancer Cells: Molecular Modeling and Biophysical Studies.Int J Mol Sci. 2025 Feb 25;26(5):1980. doi: 10.3390/ijms26051980. Int J Mol Sci. 2025. PMID: 40076604 Free PMC article.
References
-
- Powles T., Bellmunt J., Comperat E., De Santis M., Huddart R., Loriot Y., Necchi A., Valderrama B.P., Ravaud A., Shariat S.F., et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022;33:244–258. doi: 10.1016/j.annonc.2021.11.012. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

