TET Enzymes and 5hmC Levels in Carcinogenesis and Progression of Breast Cancer: Potential Therapeutic Targets
- PMID: 38203443
- PMCID: PMC10779134
- DOI: 10.3390/ijms25010272
TET Enzymes and 5hmC Levels in Carcinogenesis and Progression of Breast Cancer: Potential Therapeutic Targets
Abstract
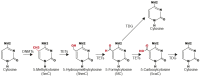
Breast Cancer (BC) was the most common female cancer in incidence and mortality worldwide in 2020. Similarly, BC was the top female cancer in the USA in 2022. Risk factors include earlier age at menarche, oral contraceptive use, hormone replacement therapy, high body mass index, and mutations in BRCA1/2 genes, among others. BC is classified into Luminal A, Luminal B, HER2-like, and Basal-like subtypes. These BC subtypes present differences in gene expression signatures, which can impact clinical behavior, treatment response, aggressiveness, metastasis, and survival of patients. Therefore, it is necessary to understand the epigenetic molecular mechanism of transcriptional regulation in BC, such as DNA demethylation. Ten-Eleven Translocation (TET) enzymes catalyze the oxidation of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) on DNA, which in turn inhibits or promotes the gene expression. Interestingly, the expression of TET enzymes as well as the levels of the 5hmC epigenetic mark are altered in several types of human cancers, including BC. Several studies have demonstrated that TET enzymes and 5hmC play a key role in the regulation of gene expression in BC, directly (dependent or independent of DNA de-methylation) or indirectly (via interaction with other proteins such as transcription factors). In this review, we describe our recent understanding of the regulatory and physiological function of the TET enzymes, as well as their potential role as biomarkers in BC biology.
Keywords: 5hmC; TET1; TET2; TET3; breast cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

