Balance between Pro- and Antifibrotic Proteins in Mesenchymal Stromal Cell Secretome Fractions Revealed by Proteome and Cell Subpopulation Analysis
- PMID: 38203461
- PMCID: PMC10779358
- DOI: 10.3390/ijms25010290
Balance between Pro- and Antifibrotic Proteins in Mesenchymal Stromal Cell Secretome Fractions Revealed by Proteome and Cell Subpopulation Analysis
Abstract
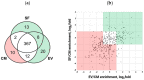
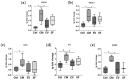
Multipotent mesenchymal stromal cells (MSCs) regulate tissue repair through paracrine activity, with secreted proteins being significant contributors. Human tissue repair commonly results in fibrosis, where fibroblast differentiation into myofibroblasts is a major cellular mechanism. MSCs' paracrine activity can inhibit fibrosis development. We previously demonstrated that the separation of MSC secretome, represented by conditioned medium (CM), into subfractions enriched with extracellular vesicles (EV) or soluble factors (SF) boosts EV and SF antifibrotic effect. This effect is realized through the inhibition of fibroblast-to-myofibroblast differentiation in vitro. To unravel the mechanisms of MSC paracrine effects on fibroblast differentiation, we performed a comparative proteomic analysis of MSC secretome fractions. We found that CM was enriched in NF-κB activators and confirmed via qPCR that CM, but not EV or SF, upregulated NF-κB target genes (COX2, IL6, etc.) in human dermal fibroblasts. Furthermore, we revealed that EV and SF were enriched in TGF-β, Notch, IGF, and Wnt pathway regulators. According to scRNAseq, 11 out of 13 corresponding genes were upregulated in a minor MSC subpopulation disappearing in profibrotic conditions. Thus, protein enrichment of MSC secretome fractions and cellular subpopulation patterns shift the balance in fibroblast-to-myofibroblast differentiation, which should be considered in studies of MSC paracrine effects and the therapeutic use of MSC secretome.
Keywords: cell differentiation; cell subpopulation; extracellular vesicles; fibroblasts; fibrosis; multipotent mesenchymal stromal cells; myofibroblasts; proteomics; secretome; single cell RNA sequencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

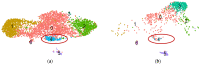
Similar articles
-
Secretome of Mesenchymal Stromal Cells Prevents Myofibroblasts Differentiation by Transferring Fibrosis-Associated microRNAs within Extracellular Vesicles.Cells. 2020 May 20;9(5):1272. doi: 10.3390/cells9051272. Cells. 2020. PMID: 32443855 Free PMC article.
-
Cytokine priming enhances the antifibrotic effects of human adipose derived mesenchymal stromal cells conditioned medium.Stem Cell Res Ther. 2024 Sep 27;15(1):329. doi: 10.1186/s13287-024-03916-9. Stem Cell Res Ther. 2024. PMID: 39334258 Free PMC article.
-
M2-Macrophage-Induced Chronic Inflammation Promotes Reversible Mesenchymal Stromal Cell Senescence and Reduces Their Anti-Fibrotic Properties.Int J Mol Sci. 2023 Dec 4;24(23):17089. doi: 10.3390/ijms242317089. Int J Mol Sci. 2023. PMID: 38069411 Free PMC article.
-
Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning.Front Immunol. 2018 Dec 4;9:2837. doi: 10.3389/fimmu.2018.02837. eCollection 2018. Front Immunol. 2018. PMID: 30564236 Free PMC article. Review.
-
Proteomic techniques for characterisation of mesenchymal stem cell secretome.Biochimie. 2013 Dec;95(12):2196-211. doi: 10.1016/j.biochi.2013.07.015. Epub 2013 Jul 20. Biochimie. 2013. PMID: 23880644 Review.
Cited by
-
Fundamental and Practical Perspectives in Regenerative Medicine.Int J Mol Sci. 2024 Oct 26;25(21):11508. doi: 10.3390/ijms252111508. Int J Mol Sci. 2024. PMID: 39519061 Free PMC article.
-
Dimension reduction, cell clustering, and cell-cell communication inference for single-cell transcriptomics with DcjComm.Genome Biol. 2024 Sep 9;25(1):241. doi: 10.1186/s13059-024-03385-6. Genome Biol. 2024. PMID: 39252099 Free PMC article.
-
Exploring Regulatory Frameworks for Exosome Therapy: Insights and Perspectives.Health Care Sci. 2025 Aug 4;4(4):299-309. doi: 10.1002/hcs2.70028. eCollection 2025 Aug. Health Care Sci. 2025. PMID: 40861511 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

