Suppression of Neuroinflammation by Coffee Component Pyrocatechol via Inhibition of NF-κB in Microglia
- PMID: 38203488
- PMCID: PMC10778612
- DOI: 10.3390/ijms25010316
Suppression of Neuroinflammation by Coffee Component Pyrocatechol via Inhibition of NF-κB in Microglia
Abstract
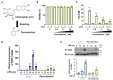
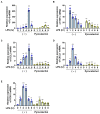
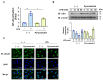
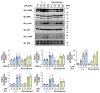
According to numerous studies, it has been epidemiologically suggested that habitual coffee intake seems to prevent the onset of neurodegenerative diseases. In this study, we hypothesized that coffee consumption suppresses neuroinflammation, which is closely related to the development of neurodegenerative diseases. Using microglial BV-2 cells, we first found that the inflammatory responses induced by lipopolysaccharide (LPS) stimulation was diminished by both coffee and decaffeinated coffee through the inhibition of an inflammation-related transcription factor, nuclear factor-κB (NF-κB). Pyrocatechol, a component of roasted coffee produced by the thermal decomposition of chlorogenic acid, also exhibited anti-inflammatory activity by inhibiting the LPS-induced activation of NF-κB. Finally, in an inflammation model using mice injected with LPS into the cerebrum, we observed that intake of pyrocatechol as well as coffee decoctions drastically suppressed the accumulation of microglia and the expression of interleukin-6 (IL-6), tumor necrosis factor α (TNFα), CCL2, and CXCL1 in the inflammatory brain. These observations strongly encourage us to hypothesize that the anti-inflammatory activity of pyrocatechol as well as coffee decoction would be useful for the suppression of neurodegeneration and the prevention of the onsets of Alzheimer's (AD) and Perkinson's diseases (PD).
Keywords: NF-κB; coffee; microglia; neuroinflammation; pyrocatechol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Pyrocatechol, a component of coffee, suppresses LPS-induced inflammatory responses by inhibiting NF-κB and activating Nrf2.Sci Rep. 2020 Feb 13;10(1):2584. doi: 10.1038/s41598-020-59380-x. Sci Rep. 2020. PMID: 32054966 Free PMC article.
-
Magnesium Lithospermate B Suppresses Lipopolysaccharide-Induced Neuroinflammation in BV2 Microglial Cells and Attenuates Neurodegeneration in Lipopolysaccharide-Injected Mice.J Mol Neurosci. 2018 Jan;64(1):80-92. doi: 10.1007/s12031-017-1007-9. Epub 2017 Dec 1. J Mol Neurosci. 2018. PMID: 29196883
-
Z-guggulsterone negatively controls microglia-mediated neuroinflammation via blocking IκB-α-NF-κB signals.Neurosci Lett. 2016 Apr 21;619:34-42. doi: 10.1016/j.neulet.2016.02.021. Epub 2016 Feb 12. Neurosci Lett. 2016. PMID: 26879835
-
Coffee, antioxidants, and brain inflammation.Prog Brain Res. 2024;289:123-150. doi: 10.1016/bs.pbr.2024.06.005. Epub 2024 Jul 3. Prog Brain Res. 2024. PMID: 39168577 Review.
-
Having a Coffee Break: The Impact of Caffeine Consumption on Microglia-Mediated Inflammation in Neurodegenerative Diseases.Mediators Inflamm. 2017;2017:4761081. doi: 10.1155/2017/4761081. Epub 2017 Jan 31. Mediators Inflamm. 2017. PMID: 28250576 Free PMC article. Review.
Cited by
-
Coffee as a Source of Antioxidants and an Elixir of Youth.Antioxidants (Basel). 2025 Feb 27;14(3):285. doi: 10.3390/antiox14030285. Antioxidants (Basel). 2025. PMID: 40227264 Free PMC article. Review.
-
Exploring the Neuroprotective Potential of N-Methylpyridinium against LPS-Induced Neuroinflammation: Insights from Molecular Mechanisms.Int J Mol Sci. 2024 May 30;25(11):6000. doi: 10.3390/ijms25116000. Int J Mol Sci. 2024. PMID: 38892185 Free PMC article.
-
Effect of Lactobacillus plantarum 1243 fermentation on quality properties and metabolome of Aronia melanocarpa (Michx.) elliott juice.Food Chem X. 2025 Jun 26;29:102706. doi: 10.1016/j.fochx.2025.102706. eCollection 2025 Jul. Food Chem X. 2025. PMID: 40672877 Free PMC article.
References
-
- Hoeffel G., Chen J., Lavin Y., Low D., Almeida F.F., See P., Beaudin A.E., Lum J., Low I., Forsberg E.C., et al. C-Myb (+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

