Enhancing Immunotherapy in Ovarian Cancer: The Emerging Role of Metformin and Statins
- PMID: 38203494
- PMCID: PMC10779012
- DOI: 10.3390/ijms25010323
Enhancing Immunotherapy in Ovarian Cancer: The Emerging Role of Metformin and Statins
Abstract
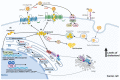
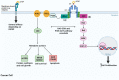
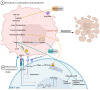
Ovarian cancer metastization is accompanied by the development of malignant ascites, which are associated with poor prognosis. The acellular fraction of this ascitic fluid contains tumor-promoting soluble factors, bioactive lipids, cytokines, and extracellular vesicles, all of which communicate with the tumor cells within this peritoneal fluid. Metabolomic profiling of ovarian cancer ascites has revealed significant differences in the pathways of fatty acids, cholesterol, glucose, and insulin. The proteins involved in these pathways promote tumor growth, resistance to chemotherapy, and immune evasion. Unveiling the key role of this liquid tumor microenvironment is crucial for discovering more efficient treatment options. This review focuses on the cholesterol and insulin pathways in ovarian cancer, identifying statins and metformin as viable treatment options when combined with standard chemotherapy. These findings are supported by clinical trials showing improved overall survival with these combinations. Additionally, statins and metformin are associated with the reversal of T-cell exhaustion, positioning these drugs as potential combinatory strategies to improve immunotherapy outcomes in ovarian cancer patients.
Keywords: T-cell exhaustion; clinical trials; drug repurposing; ovarian cancer metabolomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Common medications and survival in women with ovarian cancer: A systematic review and meta-analysis.Gynecol Oncol. 2020 Jun;157(3):678-685. doi: 10.1016/j.ygyno.2020.03.028. Epub 2020 Apr 18. Gynecol Oncol. 2020. PMID: 32317171
-
An investigation into the therapeutic effects of statins with metformin on polycystic ovary syndrome: a meta-analysis of randomised controlled trials.BMJ Open. 2015 Mar 27;5(3):e007280. doi: 10.1136/bmjopen-2014-007280. BMJ Open. 2015. PMID: 25818277 Free PMC article. Review.
-
Prognosis of ovarian cancer in women with type 2 diabetes using metformin and other forms of antidiabetic medication or statins: a retrospective cohort study.BMC Cancer. 2018 Jul 28;18(1):767. doi: 10.1186/s12885-018-4676-z. BMC Cancer. 2018. PMID: 30055585 Free PMC article.
-
Recycling the Purpose of Old Drugs to Treat Ovarian Cancer.Int J Mol Sci. 2020 Oct 20;21(20):7768. doi: 10.3390/ijms21207768. Int J Mol Sci. 2020. PMID: 33092251 Free PMC article. Review.
-
Use of "Repurposed" Drugs in the Treatment of Epithelial Ovarian Cancer: A Systematic Review.Am J Clin Oncol. 2022 Apr 1;45(4):168-174. doi: 10.1097/COC.0000000000000900. Am J Clin Oncol. 2022. PMID: 35320817
Cited by
-
Metformin induces tumor immunogenic cell death in ovarian cancer by activating AMPK pathway.Transl Oncol. 2024 Sep;47:102052. doi: 10.1016/j.tranon.2024.102052. Epub 2024 Jul 8. Transl Oncol. 2024. PMID: 38981246 Free PMC article.
-
A Narrative Review: Repurposing Metformin as a Potential Therapeutic Agent for Oral Cancer.Cancers (Basel). 2024 Aug 29;16(17):3017. doi: 10.3390/cancers16173017. Cancers (Basel). 2024. PMID: 39272875 Free PMC article. Review.
-
O-GlcNAcylation in ovarian tumorigenesis and its therapeutic implications.Transl Oncol. 2025 Jan;51:102220. doi: 10.1016/j.tranon.2024.102220. Epub 2024 Nov 30. Transl Oncol. 2025. PMID: 39616984 Free PMC article. Review.
-
Molecular Mechanisms Underlying the Anticancer Properties of Pitavastatin against Cervical Cancer Cells.Int J Mol Sci. 2024 Jul 19;25(14):7915. doi: 10.3390/ijms25147915. Int J Mol Sci. 2024. PMID: 39063157 Free PMC article.
-
Mechanical cues rewire lipid metabolism and support chemoresistance in epithelial ovarian cancer cell lines OVCAR3 and SKOV3.Cell Commun Signal. 2025 Apr 22;23(1):193. doi: 10.1186/s12964-025-02144-9. Cell Commun Signal. 2025. PMID: 40264231 Free PMC article.
References
-
- Monavarian M., Elhaw A.T., Tang P.W., Javed Z., Shonibare Z., Scalise C.B., Arend R., Jolly M.K., Sewell- Loftin M.K., Hempel N., et al. Emerging Perspectives on Growth Factor Metabolic Relationships in the Ovarian Cancer Ascites Environment. Semin. Cancer Biol. 2022;86:709–719. doi: 10.1016/j.semcancer.2022.03.004. - DOI - PMC - PubMed
-
- Krugmann J., Schwarz C.L., Melcher B., Sterlacci W., Ozalinskaite A., Lermann J., Agaimy A., Vieth M. Malignant Ascites Occurs Most Often in Patients with High-Grade Serous Papillary Ovarian Cancer at Initial Diagnosis: A Retrospective Analysis of 191 Women Treated at Bayreuth Hospital, 2006–2015. Arch. Gynecol. Obstet. 2019;299:515–523. doi: 10.1007/s00404-018-4952-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2020.00185.CEECIND/Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior (FCT)
- 2021.05081.BD/Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior (FCT)
- UIDB/50026/2020/Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior (FCT)
- UIDP/50026/2020/Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior (FCT)
LinkOut - more resources
Full Text Sources
Medical

