Studying the Roles of the Renin-Angiotensin System in Accelerating the Disease of High-Fat-Diet-Induced Diabetic Nephropathy in a db/db and ACE2 Double-Gene-Knockout Mouse Model
- PMID: 38203500
- PMCID: PMC10779113
- DOI: 10.3390/ijms25010329
Studying the Roles of the Renin-Angiotensin System in Accelerating the Disease of High-Fat-Diet-Induced Diabetic Nephropathy in a db/db and ACE2 Double-Gene-Knockout Mouse Model
Abstract
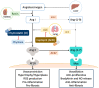
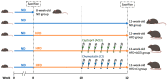
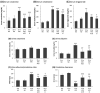
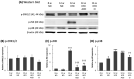
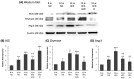
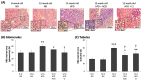
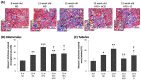
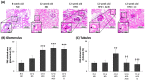
Diabetic nephropathy (DN) is a crucial metabolic health problem. The renin-angiotensin system (RAS) is well known to play an important role in DN. Abnormal RAS activity can cause the over-accumulation of angiotensin II (Ang II). Angiotensin-converting enzyme inhibitor (ACEI) administration has been proposed as a therapy, but previous studies have also indicated that chymase, the enzyme that hydrolyzes angiotensin I to Ang II in an ACE-independent pathway, may play an important role in the progression of DN. Therefore, this study established a model of severe DN progression in a db/db and ACE2 KO mouse model (db and ACE2 double-gene-knockout mice) to explore the roles of RAS factors in DNA and changes in their activity after short-term (only 4 weeks) feeding of a high-fat diet (HFD) to 8-week-old mice. The results indicate that FD-fed db/db and ACE2 KO mice fed an HFD represent a good model for investigating the role of RAS in DN. An HFD promotes the activation of MAPK, including p-JNK and p-p38, as well as the RAS signaling pathway, leading to renal damage in mice. Blocking Ang II/AT1R could alleviate the progression of DN after administration of ACEI or chymase inhibitor (CI). Both ACE and chymase are highly involved in Ang II generation in HFD-induced DN; therefore, ACEI and CI are potential treatments for DN.
Keywords: angiotensin converting enzyme II (ACE2); chymase; diabetic nephropathy; high-fat-diet; renin angiotensin system.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

