CRISPR/Cas9 as a New Antiviral Strategy for Treating Hepatitis Viral Infections
- PMID: 38203503
- PMCID: PMC10779197
- DOI: 10.3390/ijms25010334
CRISPR/Cas9 as a New Antiviral Strategy for Treating Hepatitis Viral Infections
Abstract
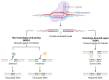
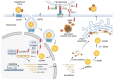
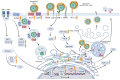
Hepatitis is an inflammatory liver disease primarily caused by hepatitis A (HAV), B (HBV), C (HCV), D (HDV), and E (HEV) viruses. The chronic forms of hepatitis resulting from HBV and HCV infections can progress to cirrhosis or hepatocellular carcinoma (HCC), while acute hepatitis can lead to acute liver failure, sometimes resulting in fatality. Viral hepatitis was responsible for over 1 million reported deaths annually. The treatment of hepatitis caused by viral infections currently involves the use of interferon-α (IFN-α), nucleoside inhibitors, and reverse transcriptase inhibitors (for HBV). However, these methods do not always lead to a complete cure for viral infections, and chronic forms of the disease pose significant treatment challenges. These facts underscore the urgent need to explore novel drug developments for the treatment of viral hepatitis. The discovery of the CRISPR/Cas9 system and the subsequent development of various modifications of this system have represented a groundbreaking advance in the quest for innovative strategies in the treatment of viral infections. This technology enables the targeted disruption of specific regions of the genome of infectious agents or the direct manipulation of cellular factors involved in viral replication by introducing a double-strand DNA break, which is targeted by guide RNA (spacer). This review provides a comprehensive summary of our current knowledge regarding the application of the CRISPR/Cas system in the regulation of viral infections caused by HAV, HBV, and HCV. It also highlights new strategies for drug development aimed at addressing both acute and chronic forms of viral hepatitis.
Keywords: CRISPR/Cas; HAV; HBV; HCV; hepatitis; replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hepatitis A. [(accessed on 20 February 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-a.
-
- Hepatitis B. [(accessed on 26 January 2021)]. Available online: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b.
-
- Hepatitis C. [(accessed on 1 February 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c.
-
- Hepatitis E. [(accessed on 19 February 2021)]. Available online: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-e.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

