Sandwich ELISA for the Quantification of Nucleocapsid Protein of SARS-CoV-2 Based on Polyclonal Antibodies from Two Different Species
- PMID: 38203504
- PMCID: PMC10778659
- DOI: 10.3390/ijms25010333
Sandwich ELISA for the Quantification of Nucleocapsid Protein of SARS-CoV-2 Based on Polyclonal Antibodies from Two Different Species
Abstract
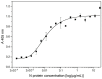
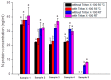
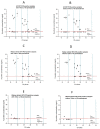
In this study, a cost-effective sandwich ELISA test, based on polyclonal antibodies, for routine quantification SARS-CoV-2 nucleocapsid (N) protein was developed. The recombinant N protein was produced and used for the production of mice and rabbit antisera. Polyclonal N protein-specific antibodies served as capture and detection antibodies. The prototype ELISA has LOD 0.93 ng/mL and LOQ 5.3 ng/mL, with a linear range of 1.52-48.83 ng/mL. N protein heat pretreatment (56 °C, 1 h) decreased, while pretreatment with 1% Triton X-100 increased analytical ELISA sensitivity. The diagnostic specificity of ELISA was 100% (95% CI, 91.19-100.00%) and sensitivity was 52.94% (95% CI, 35.13-70.22%) compared to rtRT-PCR (Ct < 40). Profoundly higher sensitivity was obtained using patient samples mostly containing Wuhan-similar variants (Wuhan, alpha, and delta), 62.50% (95% CI, 40.59 to 81.20%), in comparison to samples mostly containing Wuhan-distant variants (Omicron) 30.00% (6.67-65.25%). The developed product has relatively high diagnostic sensitivity in relation to its analytical sensitivity due to the usage of polyclonal antibodies from two species, providing a wide repertoire of antibodies against multiple N protein epitopes. Moreover, the fast, simple, and inexpensive production of polyclonal antibodies, as the most expensive assay components, would result in affordable antigen tests.
Keywords: COVID-19 diagnosis; ELISA; antigen test; nucleocapsid protein; polyclonal antibodies.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
[Development and application of triple antibodies-based sandwich ELISA for detecting nucleocapsid protein of SARS-associated coronavirus].Zhonghua Liu Xing Bing Xue Za Zhi. 2005 Apr;26(4):277-81. Zhonghua Liu Xing Bing Xue Za Zhi. 2005. PMID: 15941537 Chinese.
-
Development of a recombinant nucleocapsid protein-based ELISA for the detection of IgM and IgG antibodies to SARS-CoV-2.Biotechnol Appl Biochem. 2022 Dec;69(6):2592-2598. doi: 10.1002/bab.2308. Epub 2022 Jan 13. Biotechnol Appl Biochem. 2022. PMID: 34965611 Free PMC article.
-
Usefulness of ELISA Using Total Antibody against Plant-Expressed Recombinant Nucleocapsid Protein of SARS-CoV-2.Microbiol Spectr. 2021 Dec 22;9(3):e0067221. doi: 10.1128/Spectrum.00672-21. Epub 2021 Nov 24. Microbiol Spectr. 2021. PMID: 34817278 Free PMC article.
-
Production of a Monoclonal Antibody to the Nucleocapsid Protein of SARS-CoV-2 and Its Application to ELISA-Based Detection Methods with Broad Specificity by Combined Use of Detector Antibodies.Viruses. 2022 Dec 21;15(1):28. doi: 10.3390/v15010028. Viruses. 2022. PMID: 36680068 Free PMC article.
-
Diagnostic performance of a novel antigen-capture ELISA for the detection of SARS-CoV-2.Anal Biochem. 2023 Apr 1;666:115079. doi: 10.1016/j.ab.2023.115079. Epub 2023 Feb 7. Anal Biochem. 2023. PMID: 36754135 Free PMC article.
Cited by
-
Designing Sandwich ELISA with Broadly Reactive Anti-Nucleocapsid Monoclonal Antibodies to Detect Bat-Borne Merbecoviruses.Viruses. 2025 Jun 24;17(7):886. doi: 10.3390/v17070886. Viruses. 2025. PMID: 40733504 Free PMC article.
-
Targeting of the IL-33/Wnt axis restricts breast cancer stemness and metastasis.Sci Rep. 2025 May 25;15(1):18172. doi: 10.1038/s41598-025-03260-9. Sci Rep. 2025. PMID: 40414980 Free PMC article.
-
Establishment of a Sandwich ELISA for Detection of Pan-Merbecoviruses.Pathogens. 2025 Jun 19;14(6):605. doi: 10.3390/pathogens14060605. Pathogens. 2025. PMID: 40559613 Free PMC article.
References
-
- Hasanoglu I., Korukluoglu G., Asilturk D., Cosgun Y., Kalem A.K., Altas A.B., Kayaaslan B., Eser F., Kuzucu E.A., Guner R. Higher Viral Loads in Asymptomatic COVID-19 Patients Might Be the Invisible Part of the Iceberg. Infection. 2021;49:117–126. doi: 10.1007/s15010-020-01548-8. - DOI - PMC - PubMed
-
- Teymouri M., Mollazadeh S., Mortazavi H., Naderi Ghale-noie Z., Keyvani V., Aghababaei F., Hamblin M.R., Abbaszadeh-Goudarzi G., Pourghadamyari H., Hashemian S.M.R., et al. Pathology Research and Practice. Elsevier; Amsterdam, The Netherlands: 2021. Recent Advances and Challenges of RT-PCR Tests for the Diagnosis of COVID-19. - DOI - PMC - PubMed
-
- Tugaeva K.V., Hawkins D.E.D.P., Smith J.L.R., Bayfield O.W., Ker D.S., Sysoev A.A., Klychnikov O.I., Antson A.A., Sluchanko N.N. The Mechanism of SARS-CoV-2 Nucleocapsid Protein Recognition by the Human 14-3-3 Proteins: SARS-CoV-2 N Association with Host 14-3-3 Proteins. J. Mol. Biol. 2021;433:166875. doi: 10.1016/j.jmb.2021.166875. - DOI - PMC - PubMed
-
- Hillig T., Kristensen J.R., Brasen C.L., Brandslund I., Olsen D.A., Davidsen C., Madsen J.S., Jensen C.A., Hansen Y.B.L., Friis-Hansen L. Sensitivity and Performance of Three Novel Quantitative Assays of SARS-CoV-2 Nucleoprotein in Blood. Sci. Rep. 2023;13:2868. doi: 10.1038/s41598-023-29973-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

