Polyacrylic-Coated Solid Nanoparticles Increase the Aquaporin Permeability to Hydrogen Peroxide
- PMID: 38203543
- PMCID: PMC10778986
- DOI: 10.3390/ijms25010372
Polyacrylic-Coated Solid Nanoparticles Increase the Aquaporin Permeability to Hydrogen Peroxide
Abstract
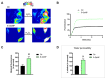
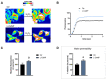
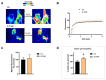
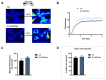
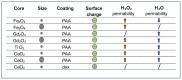
Aquaporins (AQPs) allow the diffusion of hydrogen peroxide (H2O2) and act as ROS scavenging systems, which are important for controlling the redox state of cells. Recently, cerium oxide nanoparticles were found to increase the water and H2O2 permeability by modulating AQPs. To further analyze the action of nanoparticles (NPs) on AQP, we examined the effect of the NPs presenting different core compositions (CeO2, Gd2O3, Fe3O4, and TiO2), hydrodynamic sizes, and surface functionalization. The NPs produced an increase in H2O and H2O2 permeability as a general trend. The hydrodynamic sizes of the NPs in the range of 22-100 nm did not produce any significant effect. The chemical nature of the NPs' core did not modify the effect and its intensity. On the other hand, the NPs' functionalized surface plays a major role in influencing both water and H2O2 permeability. The results suggest that NPs can play a significant role in controlling oxidative stress in cells and might represent an innovative approach in the treatment of a number of pathologies associated with an increased oxidative status.
Keywords: CeO2NP; Fe3O4NP; Gd2O3NP; HeLa; HyPer7 biosensor; TiO2NP; oxidative stress; peroxiporin; water channels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Cerium Oxide Nanoparticles Regulate Oxidative Stress in HeLa Cells by Increasing the Aquaporin-Mediated Hydrogen Peroxide Permeability.Int J Mol Sci. 2022 Sep 16;23(18):10837. doi: 10.3390/ijms231810837. Int J Mol Sci. 2022. PMID: 36142747 Free PMC article.
-
Sigma-1 Receptor Agonists Acting on Aquaporin-Mediated H2O2 Permeability: New Tools for Counteracting Oxidative Stress.Int J Mol Sci. 2021 Sep 10;22(18):9790. doi: 10.3390/ijms22189790. Int J Mol Sci. 2021. PMID: 34575952 Free PMC article.
-
Structural determinants of the hydrogen peroxide permeability of aquaporins.FEBS J. 2014 Feb;281(3):647-56. doi: 10.1111/febs.12653. Epub 2013 Dec 13. FEBS J. 2014. PMID: 24286224
-
Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide.Biochim Biophys Acta. 2014 May;1840(5):1596-604. doi: 10.1016/j.bbagen.2013.09.017. Epub 2013 Sep 20. Biochim Biophys Acta. 2014. PMID: 24060746 Review.
-
The Multifaceted Role of Aquaporin-9 in Health and Its Potential as a Clinical Biomarker.Biomolecules. 2022 Jun 27;12(7):897. doi: 10.3390/biom12070897. Biomolecules. 2022. PMID: 35883453 Free PMC article. Review.
Cited by
-
A PVP-stabilized cerium oxide-platinum nanocomposite synthesized in TEG: pro-/antioxidant activities.Nanoscale Adv. 2025 Jan 17;7(6):1686-1697. doi: 10.1039/d4na00857j. eCollection 2025 Mar 11. Nanoscale Adv. 2025. PMID: 39898281 Free PMC article.
-
Peroxiporins and Oxidative Stress: Promising Targets to Tackle Inflammation and Cancer.Int J Mol Sci. 2024 Aug 1;25(15):8381. doi: 10.3390/ijms25158381. Int J Mol Sci. 2024. PMID: 39125952 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

