The Bmtret 1 Gene Family and Its Potential Role in Response to BmNPV Stress in Bombyx mori
- PMID: 38203572
- PMCID: PMC10779185
- DOI: 10.3390/ijms25010402
The Bmtret 1 Gene Family and Its Potential Role in Response to BmNPV Stress in Bombyx mori
Abstract
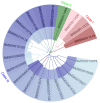
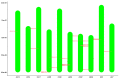
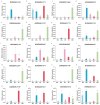
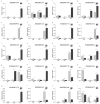
Trehalose is a non-reducing disaccharide and participates in physiological activities such as organ formation, energy metabolism, and stress resistance in insects. The Bmtret1 gene family is mainly involved in in the sugar metabolism of silkworm. In the present study, phylogenetic analysis divided 21 Bmtret1 orthologs into three clades. These genes are equally distributed on the nine chromosomes. The cis-elements in the promoter regions of Bmtret1s indicated the possible function of Bmtret1s in response to hormones and environmental stimulus. The qPCR analysis showed the significantly different expression levels of Bmtret1s in different tissues and organs, indicating possible functional divergence. In addition, most Bmtret1s showed disturbed expression levels in response to silkworm nuclear polyhedrosis virus (BmNPV) stresses. Our results provide a clue for further functional dissection of the Tret1s in Bombyx mori and implicate them as potential regulators of antiviral responses.
Keywords: BmNPV resistance; Bmtret1; Bombyx mori; bioinformatics analysis; viral replication.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Trehalose hydrolysis and transport-related genes promote Bombyx mori nucleopolyhedrovirus proliferation through the phosphoinositide 3-kinase-Akt signalling pathway in BmN cell.Dev Comp Immunol. 2023 Mar;140:104625. doi: 10.1016/j.dci.2022.104625. Epub 2022 Dec 23. Dev Comp Immunol. 2023. PMID: 36572165
-
Genome-wide identification, characterization of sugar transporter genes in the silkworm Bombyx mori and role in Bombyx mori nucleopolyhedrovirus (BmNPV) infection.Gene. 2016 Apr 1;579(2):162-71. doi: 10.1016/j.gene.2015.12.057. Epub 2015 Dec 29. Gene. 2016. PMID: 26743125
-
Label-free proteomic analysis of silkworm midgut infected by Bombyx mori nuclear polyhedrosis virus.J Proteomics. 2019 May 30;200:40-50. doi: 10.1016/j.jprot.2019.03.011. Epub 2019 Mar 20. J Proteomics. 2019. PMID: 30904731
-
The progress and future of enhancing antiviral capacity by transgenic technology in the silkworm Bombyx mori.Insect Biochem Mol Biol. 2014 May;48:1-7. doi: 10.1016/j.ibmb.2014.02.003. Epub 2014 Feb 20. Insect Biochem Mol Biol. 2014. PMID: 24561307 Review.
-
Discovery of anti-viral molecules and their vital functions in Bombyx mori.J Invertebr Pathol. 2018 May;154:12-18. doi: 10.1016/j.jip.2018.02.012. Epub 2018 Feb 14. J Invertebr Pathol. 2018. PMID: 29453967 Review.
Cited by
-
Identification of Hub Genes and Prediction of Targeted Drugs for Rheumatoid Arthritis and Idiopathic Pulmonary Fibrosis.Biochem Genet. 2024 Dec;62(6):5157-5178. doi: 10.1007/s10528-023-10650-z. Epub 2024 Feb 9. Biochem Genet. 2024. PMID: 38334875
References
-
- Luan J.B., Li J.M., Varela N., Wang Y.L., Li F.F., Bao Y.Y., Zhang C.X., Liu S.S., Wang X.W. Global analysis of the transcriptional response of whitefly to tomato yellow leaf curl China virus reveals the relationship of coevolved adaptations. J. Virol. 2011;85:3330–3340. doi: 10.1128/JVI.02507-10. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

