Nephrotoxicity in the Age of Immune Checkpoint Inhibitors: Mechanisms, Diagnosis, and Management
- PMID: 38203586
- PMCID: PMC10778678
- DOI: 10.3390/ijms25010414
Nephrotoxicity in the Age of Immune Checkpoint Inhibitors: Mechanisms, Diagnosis, and Management
Abstract
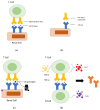
Immune checkpoint inhibitors (ICI) revolutionized cancer therapy by augmenting anti-tumor immunity via cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death-1/programmed death-ligand 1 (PD-1/PD-L1). However, this breakthrough is accompanied by immune-related adverse effects (irAEs), including renal complications. ICI-related nephritis involves complex mechanisms like auto-reactive T cells, auto-antibodies, reactivation of drug-specific T cells, and cytokine-driven inflammation culminating in AKI. ICI-AKI typically manifests weeks to months into treatment, often with other irAEs. Timely detection relies on monitoring creatinine levels and urine characteristics. Biomarkers, like soluble interleukin-2 receptor (sIL-2R) and urine cytokine levels, provide non-invasive insights, while renal biopsy remains the gold standard for confirmation. Management of ICI-AKI requires a balance between discontinuing ICI therapy and prompt immunosuppressive intervention, typically with corticosteroids. Some cases permit ICI therapy resumption, but varying renal recovery rates highlight the importance of vigilant monitoring and effective therapy. Beyond its clinical implications, the potential of irAEs to predict positive treatment responses in certain cancers raises intriguing questions. Data on nephritis-treatment response links are limited, and ongoing research explores this complex interaction. In summary, ICI therapy's transformative impact on cancer treatment is counterbalanced by irAEs, including nephritis. Early recognition and management are vital, with ongoing research refining diagnostic and treatment strategies.
Keywords: ICI-related nephritis; acute kidney injury (AKI); auto-antibodies; auto-reactive T cells; biomarkers of ICI-AKI; drug-specific T cells; immune checkpoint inhibitors (ICI); management of ICI-AKI; nephritis as a predictor of treatment response; renal biopsy; renal complications.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Seethapathy H., Zhao S., Chute D.F., Zubiri L., Oppong Y., Strohbehn I., Cortazar F.B., Leaf D.E., Mooradian M.J., Villani A.-C., et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin. J. Am. Soc. Nephrol. 2019;14:1692–1700. doi: 10.2215/CJN.00990119. - DOI - PMC - PubMed
-
- Thongprayoon C., Hansrivijit P., Kovvuru K., Kanduri S.R., Torres-Ortiz A., Acharya P., Gonzalez-Suarez M.L., Kaewput W., Bathini T., Cheungpasitporn W. Diagnostics, risk factors, treatment and outcomes of acute kidney injury in a new paradigm. J. Clin. Med. 2020;9:1104. doi: 10.3390/jcm9041104. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

