The Roles of Various Immune Cell Populations in Immune Response against Helminths
- PMID: 38203591
- PMCID: PMC10778651
- DOI: 10.3390/ijms25010420
The Roles of Various Immune Cell Populations in Immune Response against Helminths
Abstract
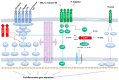
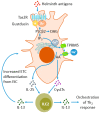
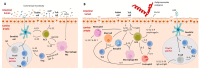
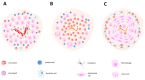
Helminths are multicellular parasites that are a substantial problem for both human and veterinary medicine. According to estimates, 1.5 billion people suffer from their infection, resulting in decreased life quality and burdens for healthcare systems. On the other hand, these infections may alleviate autoimmune diseases and allergy symptoms. The immune system is programmed to combat infections; nevertheless, its effector mechanisms may result in immunopathologies and exacerbate clinical symptoms. This review summarizes the role of the immune response against worms, with an emphasis on the Th2 response, which is a hallmark of helminth infections. We characterize non-immune cells (enteric tuft cells-ETCs) responsible for detecting parasites, as well as the role of hematopoietic-derived cells (macrophages, basophils, eosinophils, neutrophils, innate lymphoid cells group 2-ILC2s, mast cells, T cells, and B cells) in initiating and sustaining the immune response, as well as the functions they play in granulomas. The aim of this paper is to review the existing knowledge regarding the immune response against helminths, to attempt to decipher the interactions between cells engaged in the response, and to indicate the gaps in the current knowledge.
Keywords: B cells; ETC; ILC2; T cells; basophils; eosinophils; granuloma; helminths; immune response; macrophages; neutrophils.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
ILC2s-Trailblazers in the Host Response Against Intestinal Helminths.Front Immunol. 2019 Apr 4;10:623. doi: 10.3389/fimmu.2019.00623. eCollection 2019. Front Immunol. 2019. PMID: 31019505 Free PMC article. Review.
-
Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses.Cell Mol Immunol. 2019 Mar;16(3):225-235. doi: 10.1038/s41423-019-0210-8. Epub 2019 Feb 21. Cell Mol Immunol. 2019. PMID: 30792500 Free PMC article. Review.
-
Group 2 ILCs: A way of enhancing immune protection against human helminths?Parasite Immunol. 2018 Feb;40(2):e12450. doi: 10.1111/pim.12450. Epub 2017 Jul 12. Parasite Immunol. 2018. PMID: 28626924 Free PMC article. Review.
-
[Role of type 2 innate lymphoid cells in helminth infections: a review].Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2022 Aug 24;35(2):184-190. doi: 10.16250/j.32.1374.2022041. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2022. PMID: 37253569 Review. Chinese.
-
Communication is key: Innate immune cells regulate host protection to helminths.Front Immunol. 2022 Sep 26;13:995432. doi: 10.3389/fimmu.2022.995432. eCollection 2022. Front Immunol. 2022. PMID: 36225918 Free PMC article. Review.
Cited by
-
miRNA let-7-5p present in the extracellular vesicles of Trichinella spiralis newborn larvae inhibits the function of M1-type RAW264.7 macrophages by targeting C/EBPδ.Parasit Vectors. 2025 Jun 1;18(1):199. doi: 10.1186/s13071-025-06802-2. Parasit Vectors. 2025. PMID: 40452071 Free PMC article.
-
Unraveling the Dynamics of Human Filarial Infections: Immunological Responses, Host Manifestations, and Pathogen Biology.Pathogens. 2025 Feb 25;14(3):223. doi: 10.3390/pathogens14030223. Pathogens. 2025. PMID: 40137708 Free PMC article. Review.
References
-
- [(accessed on 16 November 2023)]; Available online: https://www.cdc.gov/parasites/about.html.
-
- Gross M.D. Chasing Snails: Anti-Schistosomiasis Campaigns in the People’s Republic of China. University of California; San Diego, CA, USA: 2010. pp. 64–65.
-
- Berry-Cabán C.S. Return of the God of plague: Schistosomiasis in China. J. Rural Trop. Public Health. 2007;6:45–53.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

