SMC5 Plays Independent Roles in Congenital Heart Disease and Neurodevelopmental Disability
- PMID: 38203602
- PMCID: PMC10779392
- DOI: 10.3390/ijms25010430
SMC5 Plays Independent Roles in Congenital Heart Disease and Neurodevelopmental Disability
Abstract
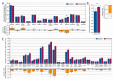
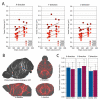
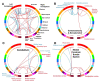
Up to 50% of patients with severe congenital heart disease (CHD) develop life-altering neurodevelopmental disability (NDD). It has been presumed that NDD arises in CHD cases because of hypoxia before, during, or after cardiac surgery. Recent studies detected an enrichment in de novo mutations in CHD and NDD, as well as significant overlap between CHD and NDD candidate genes. However, there is limited evidence demonstrating that genes causing CHD can produce NDD independent of hypoxia. A patient with hypoplastic left heart syndrome and gross motor delay presented with a de novo mutation in SMC5. Modeling mutation of smc5 in Xenopus tropicalis embryos resulted in reduced heart size, decreased brain length, and disrupted pax6 patterning. To evaluate the cardiac development, we induced the conditional knockout (cKO) of Smc5 in mouse cardiomyocytes, which led to the depletion of mature cardiomyocytes and abnormal contractility. To test a role for Smc5 specifically in the brain, we induced cKO in the mouse central nervous system, which resulted in decreased brain volume, and diminished connectivity between areas related to motor function but did not affect vascular or brain ventricular volume. We propose that genetic factors, rather than hypoxia alone, can contribute when NDD and CHD cases occur concurrently.
Keywords: cardiomyocytes; congenital heart disease; functional MRI; functional connectivity; hypoplastic left heart syndrome; neurodevelopment; structural maintenance of chromosomes.
Conflict of interest statement
Mustafa Khokha is a founder of Victory Genomics, Inc., and Philip Jordan is on the scientific advisory board of Gameto, Inc. All the other authors have no conflicts of interest to disclose. The opinions and assertions expressed herein are those of the author(s) and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences or the Department of Defense.
Figures




Similar articles
-
De novo damaging variants associated with congenital heart diseases contribute to the connectome.Sci Rep. 2020 Apr 27;10(1):7046. doi: 10.1038/s41598-020-63928-2. Sci Rep. 2020. PMID: 32341405 Free PMC article.
-
Genetics of congenital heart disease.Clin Chim Acta. 2024 Jan 1;552:117683. doi: 10.1016/j.cca.2023.117683. Epub 2023 Nov 28. Clin Chim Acta. 2024. PMID: 38030030 Review.
-
Genetic burden and associations with adverse neurodevelopment in neonates with congenital heart disease.Am Heart J. 2018 Jul;201:33-39. doi: 10.1016/j.ahj.2018.03.021. Epub 2018 Apr 5. Am Heart J. 2018. PMID: 29910053
-
De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies.Science. 2015 Dec 4;350(6265):1262-6. doi: 10.1126/science.aac9396. Science. 2015. PMID: 26785492 Free PMC article.
-
Biological and structural phenotypes associated with neurodevelopmental outcomes in congenital heart disease.Transl Pediatr. 2023 Apr 29;12(4):768-786. doi: 10.21037/tp-22-687. Epub 2023 Apr 27. Transl Pediatr. 2023. PMID: 37181016 Free PMC article. Review.
Cited by
-
Exosomal biomarkers and immune checkpoint inhibitors: advancements and future prospects.Cancer Cell Int. 2025 Jun 24;25(1):234. doi: 10.1186/s12935-025-03806-x. Cancer Cell Int. 2025. PMID: 40555997 Free PMC article. Review.
-
Single cell transcription revealing key transcription factors in embryonic kidney development.Mol Cell Biochem. 2025 May 20. doi: 10.1007/s11010-025-05307-x. Online ahead of print. Mol Cell Biochem. 2025. PMID: 40392427
-
Regulation of p53 by the mitotic surveillance/stopwatch pathway: implications in neurodevelopment and cancer.Front Cell Dev Biol. 2024 Sep 27;12:1451274. doi: 10.3389/fcell.2024.1451274. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39398482 Free PMC article. Review.
References
-
- Verrall C.E., Blue G.M., Loughran-Fowlds A., Kasparian N., Gecz J., Walker K., Dunwoodie S.L., Cordina R., Sholler G., Badawi N., et al. ‘Big issues’ in neurodevelopment for children and adults with congenital heart disease. Open Heart. 2019;6:e000998. doi: 10.1136/openhrt-2018-000998. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

