Sequential Therapy with Ropeginterferon Alfa-2b and Anti-Programmed Cell Death 1 Antibody for Inhibiting the Recurrence of Hepatitis B-Related Hepatocellular Carcinoma: From Animal Modeling to Phase I Clinical Results
- PMID: 38203603
- PMCID: PMC10778875
- DOI: 10.3390/ijms25010433
Sequential Therapy with Ropeginterferon Alfa-2b and Anti-Programmed Cell Death 1 Antibody for Inhibiting the Recurrence of Hepatitis B-Related Hepatocellular Carcinoma: From Animal Modeling to Phase I Clinical Results
Abstract
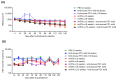
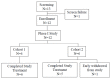
Hepatocellular carcinoma (HCC) usually recurs after curative surgical resection. Currently, no approved adjuvant therapy has been shown to reduce HCC recurrence rates. In this study, the in vivo effect of sequential combination treatment with recombinant mouse interferon-alpha (rmIFN-α) and an anti-mouse-PD1 antibody on hepatitis B virus (HBV) clearance in mice was evaluated. A Phase I clinical trial was then conducted to assess the safety, tolerability, and inhibitory activity of sequential therapy with ropeginterferon alfa-2b and nivolumab in patients with HCC recurrence who underwent curative surgery for HBV-related HCC. The animal modeling study showed that HBV suppression was significantly greater with the rmIFN-α and anti-PD1 sequential combination treatment in comparison with sole treatment with rmIFN-α or anti-PD1. In the Phase I study, eleven patients completed the sequential therapy with ropeginterferon alfa-2b every two weeks for six doses at 450 µg, followed by three doses of nivolumab every two weeks up to 0.75 mg/kg. A notable decrease in or clearance of HBV surface antigen was observed in two patients. The dose-limiting toxicity of grade 3 alanine transaminase and aspartate aminotransferase increases was observed in one patient. The maximum tolerated dose was then determined. To date, no HCC recurrence has been observed. The treatment modality was well tolerated. These data support the further clinical development of sequential combination therapy as a post-surgery prophylactic measure against the recurrence of HBV-related HCC.
Keywords: HBV-related; animal HBV model; anti-PD1 antibody; clinical trial; hepatocellular carcinoma; ropeginterferon alfa-2b.
Conflict of interest statement
Albert Qin and Chan-Yen Tsai work for the PharmaEssentia Corporation. Pei-Jer Chen has served as a consultant for the PharmaEssentia Corporation. The other authors have no conflicts of interest to declare.
Figures
References
-
- Global Burden of Disease Liver Cancer Collaboration. Akinyemiju T., Abera S., Ahmed M., Alam N., Alemayohu M.A., Allen C., Al-Raddadi R., Alvis-Guzman N., Amoako Y., et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683–1691. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical