The RyR1 P3528S Substitution Alters Mouse Skeletal Muscle Contractile Properties and RyR1 Ion Channel Gating
- PMID: 38203604
- PMCID: PMC10778724
- DOI: 10.3390/ijms25010434
The RyR1 P3528S Substitution Alters Mouse Skeletal Muscle Contractile Properties and RyR1 Ion Channel Gating
Abstract
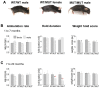
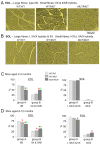
The recessive Ryanodine Receptor Type 1 (RyR1) P3527S mutation causes mild muscle weakness in patients and increased resting cytoplasmic [Ca2+] in transformed lymphoblastoid cells. In the present study, we explored the cellular/molecular effects of this mutation in a mouse model of the mutation (RyR1 P3528S). The results were obtained from 73 wild type (WT/WT), 82 heterozygous (WT/MUT) and 66 homozygous (MUT/MUT) mice with different numbers of observations in individual data sets depending on the experimental protocol. The results showed that WT/MUT and MUT/MUT mouse strength was less than that of WT/WT mice, but there was no difference between genotypes in appearance, weight, mobility or longevity. The force frequency response of extensor digitorum longus (EDL) and soleus (SOL) muscles from WT/MUT and MUT/MUT mice was shifter to higher frequencies. The specific force of EDL muscles was reduced and Ca2+ activation of skinned fibres shifted to a lower [Ca2+], with an increase in type I fibres in EDL muscles and in mixed type I/II fibres in SOL muscles. The relative activity of RyR1 channels exposed to 1 µM cytoplasmic Ca2+ was greater in WT/MUT and MUT/MUT mice than in WT/WT mice. We suggest the altered RyR1 activity due to the P2328S substitution could increase resting [Ca2+] in muscle fibres, leading to changes in fibre type and contractile properties.
Keywords: Ca2+ activation of skinned muscle fibres; RyR1 P3528S substitution; fibre-type composition; mouse model; muscle contractile properties; ryanodine receptor; single ryanodine receptor activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

