Antibody Response to HERV-K and HERV-W Envelope Epitopes in Patients with Myasthenia Gravis
- PMID: 38203616
- PMCID: PMC10778599
- DOI: 10.3390/ijms25010446
Antibody Response to HERV-K and HERV-W Envelope Epitopes in Patients with Myasthenia Gravis
Abstract
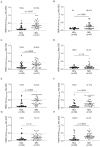
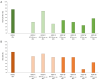
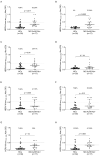
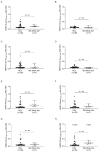
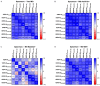
Myasthenia gravis is an antibody-mediated autoimmune neurological disorder characterized by impaired neuromuscular junction transmission, resulting in muscle weakness. Recently, the involvement of Human Endogenous Retroviruses (HERVs) in the pathophysiology of different immune-mediated and neurodegenerative diseases, such as multiple sclerosis, has been demonstrated. We aimed to investigate potential immune system involvement related to humoral responses targeting specific epitopes of HERV-K and HERV-W envelope proteins in myasthenia gravis. Myasthenia gravis patients were recruited in the Neurology Unit, while healthy controls were selected from the Blood Transfusion Center, both affiliated with AOU Sassari. Highly immunogenic antigens of HERV-K and HERV-W envelope proteins were identified using the Immune Epitope Database (IEDB) online tool. These epitopes were utilized in enzyme-linked immunosorbent assays (ELISA) to detect autoantibodies in serum directed against these sequences. The study involved 39 Healthy Donors and 47 MG patients, further categorized into subgroups based on the presence of autoantibodies: MG-AchR Ab+ (n = 17), MG-MuSK Ab+ (n = 7), double seronegative patients (MG-DSN, n = 18), MG-LRP4 Ab + (n = 4), and one patient with no antibodies data (n = 1). Our findings revealed high levels of autoantibodies in myasthenia gravis patients directed against the HERV-K-env-su(19-37), HERV-K-env-su(109-126), HERV-K-env-su(164-186), HERV-W-env(93-108), HERV-W-env(129-14), and HERV-W-env(248-262) epitopes. Notably, these results remained highly significant even when patients were subdivided into MG-AchR Ab+ and MG-DSN subgroups. Correlation analysis further revealed significant positive associations between the antibody levels against HERV-K and HERV-W families in patients, suggesting a synergistic action of the two HERVs in the pathology context since this correlation is absent in the control group. This study marks the first identification of a specific humoral response directed against defined epitopes of HERV-K and HERV-W envelope proteins in myasthenia gravis patients. These findings lay the foundation for future investigations aimed at elucidating the molecular mechanisms driving this immune response. The detection of these autoantibodies suggests the potential for novel biomarkers, especially within the MG-DSN patient subgroup, addressing the need for new biomarkers in this population.
Keywords: HERV-K; HERV-W; MG; autoantibodies; epitopes; humoral response; myasthenia gravis.
Conflict of interest statement
The authors declared no conflict of interest.
Figures

References
-
- Arru G., Sechi E., Mariotto S., Farinazzo A., Mancinelli C., Alberti D., Ferrari S., Gajofatto A., Capra R., Monaco S., et al. Antibody response against HERV-W env surface peptides differentiates multiple sclerosis and neuromyelitis optica spectrum disorder. Mult. Scler. J.-Exp. Transl. Clin. 2017;3:2055217317742425. doi: 10.1177/2055217317742425. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

