Statins-From Fungi to Pharmacy
- PMID: 38203637
- PMCID: PMC10779115
- DOI: 10.3390/ijms25010466
Statins-From Fungi to Pharmacy
Abstract
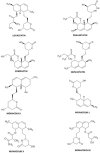
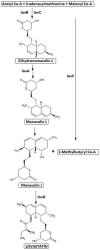
Statins have been used in the treatment of hyperlipidemia, both as monotherapy and in combination therapy. Natural fermentation processes of fungi such as Monascus spp., Penicillium spp., Aspergillus terreus, and Pleurotus ostreatus have given rise to natural statins. Compactin (mevastatin), the original naturally occurring statin, is the primary biotransformation substrate in the manufacturing process of marketed drugs. Statins are classified into natural, semi-synthetic derivatives of natural statins, and synthetic ones. Synthetic statins differ from natural statins in their structural composition, with the only common feature being the HMG-CoA-like moiety responsible for suppressing HMG-CoA reductase. Statins do not differ significantly regarding their pleiotropic and adverse effects, but their characteristics depend on their pharmacokinetic parameters and chemical properties. This paper focuses on describing the processes of obtaining natural statins, detailing the pharmacokinetics of available statins, divided into natural and synthetic, and indicating their pleiotropic effects.
Keywords: HMG-CoA reductase; cholesterol; fungi; statins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Endo A. Discovery and Development of Statins. Nat. Prod. Commun. 2017;12:1934578X1701200. doi: 10.1177/1934578X1701200801. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

