A Novel Rabbit Model of Retained Hemothorax with Pleural Organization
- PMID: 38203639
- PMCID: PMC10779131
- DOI: 10.3390/ijms25010470
A Novel Rabbit Model of Retained Hemothorax with Pleural Organization
Abstract
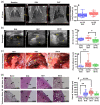
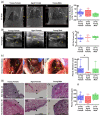
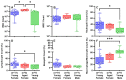
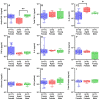
Retained hemothorax (RH) is a commonly encountered and potentially severe complication of intrapleural bleeding that can organize with lung restriction. Early surgical intervention and intrapleural fibrinolytic therapy have been advocated. However, the lack of a reliable, cost-effective model amenable to interventional testing has hampered our understanding of the role of pharmacological interventions in RH management. Here, we report the development of a new RH model in rabbits. RH was induced by sequential administration of up to three doses of recalcified citrated homologous rabbit donor blood plus thrombin via a chest tube. RH at 4, 7, and 10 days post-induction (RH4, RH7, and RH10, respectively) was characterized by clot retention, intrapleural organization, and increased pleural rind, similar to that of clinical RH. Clinical imaging techniques such as ultrasonography and computed tomography (CT) revealed the dynamic formation and resorption of intrapleural clots over time and the resulting lung restriction. RH7 and RH10 were evaluated in young (3 mo) animals of both sexes. The RH7 recapitulated the most clinically relevant RH attributes; therefore, we used this model further to evaluate the effect of age on RH development. Sanguineous pleural fluids (PFs) in the model were generally small and variably detected among different models. The rabbit model PFs exhibited a proinflammatory response reminiscent of human hemothorax PFs. Overall, RH7 results in the consistent formation of durable intrapleural clots, pleural adhesions, pleural thickening, and lung restriction. Protracted chest tube placement over 7 d was achieved, enabling direct intrapleural access for sampling and treatment. The model, particularly RH7, is amenable to testing new intrapleural pharmacologic interventions, including iterations of currently used empirically dosed agents or new candidates designed to safely and more effectively clear RH.
Keywords: coagulation; fibrinolysis; pleural fluid; pleural organization; rabbits; retained hemothorax; thoracostomy tube.
Conflict of interest statement
This work was not funded by any commercial entities. Steven Idell M.D., Ph.D. is supported by NIH, founded Lung Therapeutics, Inc. (LTI), and has equity in the company, which is commercializing single chain urokinase for empyema, has served as a paid consultant as needed for the company, and has a conflict-of-interest management plan from the University of Texas at Tyler accordingly. Andrey A. Komissarov Ph.D. and Galina Florova Ph.D. are supported by NIH and serve as co-investigators on research involving intellectual property licensed to LTI and have conflict-of-interest management plans at The University of Texas Health Science Center at Tyler (UTHSCT). Rene Girard M.S. and Krishna Sarva M.S. served as research associates on research involving intellectual property licensed to LTI and likewise had conflict-of-interest management plans at UTHSCT. The rest of the authorship has no conflicts of interest to disclose.
Figures



References
-
- Rossmann M., Altomare M., Pezzoli I., Abruzzese A., Spota A., Vettorello M., Cioffi S.P.B., Virdis F., Bini R., Chiara O., et al. Risk Factors for Retained Hemothorax after Trauma: A 10-Years Monocentric Experience from First Level Trauma Center in Italy. J. Pers. Med. 2022;12:1570. doi: 10.3390/jpm12101570. - DOI - PMC - PubMed
-
- Prakash P.S., Moore S.A., Rezende-Neto J.B., Trpcic S., Dunn J.A., Smoot B., Jenkins D.H., Cardenas T., Mukherjee K., Farnsworth J., et al. Predictors of retained hemothorax in trauma: Results of an Eastern Association for the Surgery of Trauma multi-institutional trial. J. Trauma Acute Care Surg. 2020;89:679–685. doi: 10.1097/TA.0000000000002881. - DOI - PubMed
-
- DuBose J., Inaba K., Okoye O., Demetriades D., Scalea T., O’Connor J., Menaker J., Morales C., Shiflett T., Brown C., et al. Development of posttraumatic empyema in patients with retained hemothorax: Results of a prospective, observational AAST study. J. Trauma Acute. Care Surg. 2012;73:752–757. doi: 10.1097/TA.0b013e31825c1616. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

