Soluble Forms of Immune Checkpoints and Their Ligands as Potential Biomarkers in the Diagnosis of Recurrent Pregnancy Loss-A Preliminary Study
- PMID: 38203670
- PMCID: PMC10779235
- DOI: 10.3390/ijms25010499
Soluble Forms of Immune Checkpoints and Their Ligands as Potential Biomarkers in the Diagnosis of Recurrent Pregnancy Loss-A Preliminary Study
Abstract
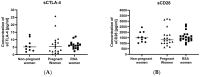
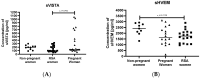
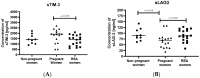
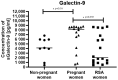
Immune checkpoints (ICPs) serve as regulatory switches on immune-competent cells. Soluble ICPs consist of fragments derived from ICP molecules typically located on cell membranes. Research has demonstrated that they perform similar functions to their membrane-bound counterparts but are directly present in the bloodstream. Effective control of the maternal immune system is vital for a successful pregnancy due to genetic differences between the mother and fetus. Abnormalities in the immune response are widely acknowledged as the primary cause of spontaneous abortions. In our research, we introduce a novel approach to understanding the immune-mediated mechanisms underlying recurrent miscarriages and explore new possibilities for diagnosing and preventing pregnancy loss. The female participants in the study were divided into three groups: RSA (recurrent spontaneous abortion), pregnant, and non-pregnant women. The analysis of soluble forms of immune checkpoints and their ligands in the serum of the study groups was conducted using the Luminex method Statistically significant differences in the concentrations of (ICPs) were observed between physiological pregnancies and the RSA group. Among patients with RSA, we noted reduced concentrations of sGalectin-9, sTIM-3, and sCD155, along with elevated concentrations of LAG-3, sCD80, and sCD86 ICPs, in comparison to physiological pregnancies. Our study indicates that sGalectin-9, TIM-3, sLAG-3, sCD80, sCD86, sVISTA, sNectin-2, and sCD155 could potentially serve as biological markers of a healthy, physiological pregnancy. These findings suggest that changes in the concentrations of soluble immune checkpoints may have the potential to act as markers for early pregnancy loss.
Keywords: CTLA-4; PD-1; RSA; TIGIT; TIM-3; VISTA; immune checkpoints; miscarriage; pregnancy loss; recurrent spontaneous abortion; soluble immune checkpoints.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures




Similar articles
-
Surface Immune Checkpoints as Potential Biomarkers in Physiological Pregnancy and Recurrent Pregnancy Loss.Int J Mol Sci. 2024 Aug 29;25(17):9378. doi: 10.3390/ijms25179378. Int J Mol Sci. 2024. PMID: 39273326 Free PMC article.
-
Differences in Immune Checkpoints Expression (TIM-3 and PD-1) on T Cells in Women with Recurrent Miscarriages-Preliminary Studies.J Clin Med. 2021 Sep 16;10(18):4182. doi: 10.3390/jcm10184182. J Clin Med. 2021. PMID: 34575293 Free PMC article.
-
The evaluation of PD-1 and Tim-3 expression besides their related miRNAs in PBMCs of women with recurrent pregnancy loss.Immunol Lett. 2024 Apr;266:106837. doi: 10.1016/j.imlet.2024.106837. Epub 2024 Jan 23. Immunol Lett. 2024. PMID: 38266686
-
Tim-3: An inhibitory immune checkpoint is associated with maternal-fetal tolerance and recurrent spontaneous abortion.Clin Immunol. 2022 Dec;245:109185. doi: 10.1016/j.clim.2022.109185. Epub 2022 Nov 11. Clin Immunol. 2022. PMID: 36372320 Review.
-
The Role of Immune Cells in Recurrent Spontaneous Abortion.Reprod Sci. 2021 Dec;28(12):3303-3315. doi: 10.1007/s43032-021-00599-y. Epub 2021 Jun 8. Reprod Sci. 2021. PMID: 34101149 Free PMC article. Review.
Cited by
-
Unveiling sialoglycans' immune mastery in pregnancy and their intersection with tumor biology.Front Immunol. 2024 Dec 20;15:1479181. doi: 10.3389/fimmu.2024.1479181. eCollection 2024. Front Immunol. 2024. PMID: 39759524 Free PMC article. Review.
-
Exploring the Immunological Aspects and Treatments of Recurrent Pregnancy Loss and Recurrent Implantation Failure.Int J Mol Sci. 2025 Feb 3;26(3):1295. doi: 10.3390/ijms26031295. Int J Mol Sci. 2025. PMID: 39941063 Free PMC article. Review.
-
Insights into Reproductive Immunology and Placental Pathology.Int J Mol Sci. 2024 Nov 12;25(22):12135. doi: 10.3390/ijms252212135. Int J Mol Sci. 2024. PMID: 39596208 Free PMC article.
-
Immune checkpoint for pregnancy.Semin Immunopathol. 2025 May 2;47(1):26. doi: 10.1007/s00281-025-01051-y. Semin Immunopathol. 2025. PMID: 40314833 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

