Transcriptomic Analysis of the Aged Nulliparous Mouse Ovary Suggests a Stress State That Promotes Pro-Inflammatory Lipid Signaling and Epithelial Cell Enrichment
- PMID: 38203684
- PMCID: PMC10779227
- DOI: 10.3390/ijms25010513
Transcriptomic Analysis of the Aged Nulliparous Mouse Ovary Suggests a Stress State That Promotes Pro-Inflammatory Lipid Signaling and Epithelial Cell Enrichment
Abstract
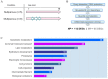
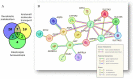
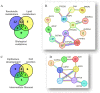
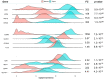
Ovarian cancer (OC) incidence and mortality peaks at post-menopause while OC risk is either reduced by parity or increased by nulliparity during fertile life. The long-term effect of nulliparity on ovarian gene expression is largely unknown. In this study, we describe a bioinformatic/data-mining analysis of 112 coding genes upregulated in the aged nulliparous (NP) mouse ovary compared to the aged multiparous one as reference. Canonical gene ontology and pathway analyses indicated a pro-oxidant, xenobiotic-like state accompanied by increased metabolism of inflammatory lipid mediators. Up-regulation of typical epithelial cell markers in the aged NP ovary was consistent with synchronized overexpression of Cldn3, Ezr, Krt7, Krt8 and Krt18 during the pre-neoplastic phase of mOSE cell cultures in a former transcriptome study. In addition, 61/112 genes were upregulated in knockout mice for Fshr and for three other tumor suppressor genes (Pten, Cdh1 and Smad3) known to regulate follicular homeostasis in the mammalian ovary. We conclude that the aged NP ovary displays a multifaceted stress state resulting from oxidative imbalance and pro-inflammatory lipid signaling. The enriched epithelial cell content might be linked to follicle depletion and is consistent with abundant clefts and cysts observed in aged human and mouse ovaries. It also suggests a mesenchymal-to-epithelial transition in the mOSE of the aged NP ovary. Our analysis suggests that in the long term, nulliparity worsens a variety of deleterious effects of aging and senescence thereby increasing susceptibility to cancer initiation in the ovary.
Keywords: age; mouse model; nulliparity; ovarian cancer; risk.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Ovarian Transcriptome of Reproductively Aged Multiparous Mice: Candidate Genes for Ovarian Cancer Protection.Biomolecules. 2020 Jan 9;10(1):113. doi: 10.3390/biom10010113. Biomolecules. 2020. PMID: 31936467 Free PMC article.
-
Parity-Dependent Hemosiderin and Lipofuscin Accumulation in the Reproductively Aged Mouse Ovary.Anal Cell Pathol (Amst). 2018 Mar 15;2018:1289103. doi: 10.1155/2018/1289103. eCollection 2018. Anal Cell Pathol (Amst). 2018. PMID: 29736365 Free PMC article.
-
KRT7 promotes epithelial‑mesenchymal transition in ovarian cancer via the TGF‑β/Smad2/3 signaling pathway.Oncol Rep. 2021 Feb;45(2):481-492. doi: 10.3892/or.2020.7886. Epub 2020 Dec 8. Oncol Rep. 2021. PMID: 33416175 Free PMC article.
-
The protective role of pregnancy in breast cancer.Breast Cancer Res. 2005;7(3):131-42. doi: 10.1186/bcr1029. Epub 2005 Apr 7. Breast Cancer Res. 2005. PMID: 15987443 Free PMC article. Review.
-
Cell biology of human ovarian surface epithelial cells and ovarian carcinogenesis.Med Electron Microsc. 2003 Jun;36(2):74-86. doi: 10.1007/s00795-002-0196-6. Med Electron Microsc. 2003. PMID: 12886939 Review.
Cited by
-
The Role of lncRNAs in the Protective Action of Tamoxifen on the Ovaries of Tumor-Bearing Rats Receiving Cyclophosphamide.Int J Mol Sci. 2024 Nov 22;25(23):12538. doi: 10.3390/ijms252312538. Int J Mol Sci. 2024. PMID: 39684249 Free PMC article.
-
Oxidative Stress, Parity History, and Remnant Follicles in the Aged Ovary: Insights on Ovarian Cancer Risk and Protection.Antioxidants (Basel). 2025 Jun 20;14(7):759. doi: 10.3390/antiox14070759. Antioxidants (Basel). 2025. PMID: 40722863 Free PMC article. Review.
References
-
- Fu Z., Brooks M.M., Irvin S., Jordan S., Aben K.K.H., Anton-Culver H., Bandera E.V., Beckmann M.W., Berchuck A., Brooks-Wilson A., et al. Lifetime ovulatory years and risk of epithelial ovarian cancer: A multinational pooled analysis. J. Natl. Cancer Inst. 2023;115:539–551. doi: 10.1093/jnci/djad011. - DOI - PMC - PubMed
-
- Agnieszka B., Brodowski J., Laszczyńska M., Słuczanowska-Głąbowska S., Rumianowski B., Rotter I., Starczewski A., Ratajczak M.Z. Immunoexpression of aromatase cytochrome P450 and 17β-hydroxysteroid dehydrogenase in women’s ovaries after menopause. J. Ovarian Res. 2014;7:52. doi: 10.1186/1757-2215-7-52. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

