Serotonergic Modulation of the Excitation/Inhibition Balance in the Visual Cortex
- PMID: 38203689
- PMCID: PMC10778629
- DOI: 10.3390/ijms25010519
Serotonergic Modulation of the Excitation/Inhibition Balance in the Visual Cortex
Abstract
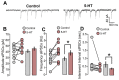
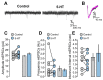
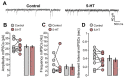
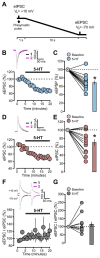
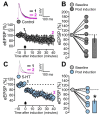
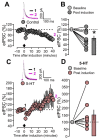
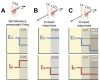
Serotonergic neurons constitute one of the main systems of neuromodulators, whose diffuse projections regulate the functions of the cerebral cortex. Serotonin (5-HT) is known to play a crucial role in the differential modulation of cortical activity related to behavioral contexts. Some features of the 5-HT signaling organization suggest its possible participation as a modulator of activity-dependent synaptic changes during the critical period of the primary visual cortex (V1). Cells of the serotonergic system are among the first neurons to differentiate and operate. During postnatal development, ramifications from raphe nuclei become massively distributed in the visual cortical area, remarkably increasing the availability of 5-HT for the regulation of excitatory and inhibitory synaptic activity. A substantial amount of evidence has demonstrated that synaptic plasticity at pyramidal neurons of the superficial layers of V1 critically depends on a fine regulation of the balance between excitation and inhibition (E/I). 5-HT could therefore play an important role in controlling this balance, providing the appropriate excitability conditions that favor synaptic modifications. In order to explore this possibility, the present work used in vitro intracellular electrophysiological recording techniques to study the effects of 5-HT on the E/I balance of V1 layer 2/3 neurons, during the critical period. Serotonergic action on the E/I balance has been analyzed on spontaneous activity, evoked synaptic responses, and long-term depression (LTD). Our results pointed out that the predominant action of 5-HT implies a reduction in the E/I balance. 5-HT promoted LTD at excitatory synapses while blocking it at inhibitory synaptic sites, thus shifting the Hebbian alterations of synaptic strength towards lower levels of E/I balance.
Keywords: 5-HT; E/I balance; visual cortex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

