Optimizing Hexose Utilization Pathways of Cupriavidus necator for Improving Growth and L-Alanine Production under Heterotrophic and Autotrophic Conditions
- PMID: 38203719
- PMCID: PMC10778655
- DOI: 10.3390/ijms25010548
Optimizing Hexose Utilization Pathways of Cupriavidus necator for Improving Growth and L-Alanine Production under Heterotrophic and Autotrophic Conditions
Abstract
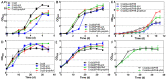
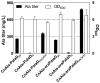
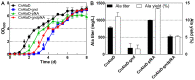
Cupriavidus necator is a versatile microbial chassis to produce high-value products. Blocking the poly-β-hydroxybutyrate synthesis pathway (encoded by the phaC1AB1 operon) can effectively enhance the production of C. necator, but usually decreases cell density in the stationary phase. To address this problem, we modified the hexose utilization pathways of C. necator in this study by implementing strategies such as blocking the Entner-Doudoroff pathway, completing the phosphopentose pathway by expressing the gnd gene (encoding 6-phosphogluconate dehydrogenase), and completing the Embden-Meyerhof-Parnas pathway by expressing the pfkA gene (encoding 6-phosphofructokinase). During heterotrophic fermentation, the OD600 of the phaC1AB1-knockout strain increased by 44.8% with pfkA gene expression alone, and by 93.1% with gnd and pfkA genes expressing simultaneously. During autotrophic fermentation, gnd and pfkA genes raised the OD600 of phaC1AB1-knockout strains by 19.4% and 12.0%, respectively. To explore the effect of the pfkA gene on the production of C. necator, an alanine-producing C. necator was constructed by expressing the NADPH-dependent L-alanine dehydrogenase, alanine exporter, and knocking out the phaC1AB1 operon. The alanine-producing strain had maximum alanine titer and yield of 784 mg/L and 11.0%, respectively. And these values were significantly improved to 998 mg/L and 13.4% by expressing the pfkA gene. The results indicate that completing the Embden-Meyerhof-Parnas pathway by expressing the pfkA gene is an effective method to improve the growth and production of C. necator.
Keywords: Cupriavidus necator; alanine; cell density; hexose utilization pathway; metabolic engineering; poly-β-hydroxybutyrate.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Ronďošová S., Legerská B., Chmelová D., Ondrejovič M., Miertuš S. Optimization of growth conditions to enhance PHA production by Cupriavidus necator. Fermentation. 2022;8:451. doi: 10.3390/fermentation8090451. - DOI
-
- Sohn Y.J., Son J., Jo S.Y., Park S.Y., Yoo J.I., Baritugo K.-A., Na J.G., Choi J.-i., Kim H.T., Joo J.C., et al. Chemoautotroph Cupriavidus necator as a potential game-changer for global warming and plastic waste problem: A review. Bioresour. Technol. 2021;340:125693. doi: 10.1016/j.biortech.2021.125693. - DOI - PubMed
-
- Tu W.L., Chu H.K., Huang C.M., Chen C.H., Ou C.M., Guo G.L. Polyhydroxyalkanoate production by Cupriavidus necator with inedible rice. BioResources. 2022;17:2202–2213. doi: 10.15376/biores.17.2.2202-2213. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

