Ectromelia Virus Affects the Formation and Spatial Organization of Adhesive Structures in Murine Dendritic Cells In Vitro
- PMID: 38203729
- PMCID: PMC10779027
- DOI: 10.3390/ijms25010558
Ectromelia Virus Affects the Formation and Spatial Organization of Adhesive Structures in Murine Dendritic Cells In Vitro
Abstract
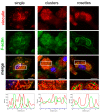
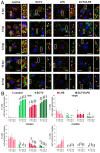
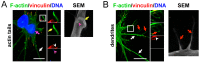
Ectromelia virus (ECTV) is a causative agent of mousepox. It provides a suitable model for studying the immunobiology of orthopoxviruses, including their interaction with the host cell cytoskeleton. As professional antigen-presenting cells, dendritic cells (DCs) control the pericellular environment, capture antigens, and present them to T lymphocytes after migration to secondary lymphoid organs. Migration of immature DCs is possible due to the presence of specialized adhesion structures, such as podosomes or focal adhesions (FAs). Since assembly and disassembly of adhesive structures are highly associated with DCs' immunoregulatory and migratory functions, we evaluated how ECTV infection targets podosomes and FAs' organization and formation in natural-host bone marrow-derived DCs (BMDC). We found that ECTV induces a rapid dissolution of podosomes at the early stages of infection, accompanied by the development of larger and wider FAs than in uninfected control cells. At later stages of infection, FAs were predominantly observed in long cellular extensions, formed extensively by infected cells. Dissolution of podosomes in ECTV-infected BMDCs was not associated with maturation and increased 2D cell migration in a wound healing assay; however, accelerated transwell migration of ECTV-infected cells towards supernatants derived from LPS-conditioned BMDCs was observed. We suggest that ECTV-induced changes in the spatial organization of adhesive structures in DCs may alter the adhesiveness/migration of DCs during some conditions, e.g., inflammation.
Keywords: Ectromelia virus; dendritic cells; focal adhesions; migration; podosomes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Dendritic cells during mousepox: The role of delayed apoptosis in the pathogenesis of infection.Microb Pathog. 2017 Aug;109:99-109. doi: 10.1016/j.micpath.2017.05.037. Epub 2017 May 26. Microb Pathog. 2017. PMID: 28554653
-
A lack of Fas/FasL signalling leads to disturbances in the antiviral response during ectromelia virus infection.Arch Virol. 2016 Apr;161(4):913-28. doi: 10.1007/s00705-015-2746-y. Epub 2016 Jan 18. Arch Virol. 2016. PMID: 26780774
-
Redundant Function of Plasmacytoid and Conventional Dendritic Cells Is Required To Survive a Natural Virus Infection.J Virol. 2015 Oct;89(19):9974-85. doi: 10.1128/JVI.01024-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202250 Free PMC article.
-
Ectromelia virus: the causative agent of mousepox.J Gen Virol. 2005 Oct;86(Pt 10):2645-2659. doi: 10.1099/vir.0.81090-0. J Gen Virol. 2005. PMID: 16186218 Review.
-
The Pathogenesis and Immunobiology of Mousepox.Adv Immunol. 2016;129:251-76. doi: 10.1016/bs.ai.2015.10.001. Epub 2015 Nov 21. Adv Immunol. 2016. PMID: 26791861 Review.
Cited by
-
Modifications of Mitochondrial Network Morphology Affect the MAVS-Dependent Immune Response in L929 Murine Fibroblasts during Ectromelia Virus Infection.Pathogens. 2024 Aug 23;13(9):717. doi: 10.3390/pathogens13090717. Pathogens. 2024. PMID: 39338909 Free PMC article.
References
-
- Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi I.D.L. Smallpox and Its Eradication. World Health Organization; Geneva, Switzerland: 1988.
-
- Ferrari G., Neukamm J., Baalsrud H.T., Breidenstein A.M., Ravinet M., Phillips C., Rühli F., Bouwman A., Schuenemann V.J. Variola Virus Genome Sequenced from an Eighteenth-Century Museum Specimen Supports the Recent Origin of Smallpox. Philos. Trans. R. Soc. B. 2020;375:20190572. doi: 10.1098/rstb.2019.0572. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

