HSA-ZW800-PEG for Enhanced Optophysical Stability and Tumor Targeting
- PMID: 38203730
- PMCID: PMC10779243
- DOI: 10.3390/ijms25010559
HSA-ZW800-PEG for Enhanced Optophysical Stability and Tumor Targeting
Abstract
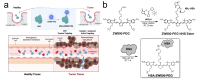
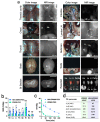
Small molecule fluorophores often face challenges such as short blood half-life, limited physicochemical and optical stability, and poor pharmacokinetics. To overcome these limitations, we conjugated the zwitterionic near-infrared fluorophore ZW800-PEG to human serum albumin (HSA), creating HSA-ZW800-PEG. This conjugation notably improves chemical, physical, and optical stability under physiological conditions, addressing issues commonly encountered with small molecules in biological applications. Additionally, the high molecular weight and extinction coefficient of HSA-ZW800-PEG enhances biodistribution and tumor targeting through the enhanced permeability and retention effect. The unique distribution and elimination dynamics, along with the significantly extended blood half-life of HSA-ZW800-PEG, contribute to improved tumor targetability in both subcutaneous and orthotopic xenograft tumor-bearing animal models. This modification not only influences the pharmacokinetic profile, affecting retention time and clearance patterns, but also enhances bioavailability for targeting tissues. Our study guides further development and optimization of targeted imaging agents and drug-delivery systems.
Keywords: NIR imaging; enhanced permeability and retention; human serum albumin; photostability; tumor targeting.
Conflict of interest statement
The authors declare no conflicts of interest. H.S.C. reports a relationship with Nawoo Vision and Ferrex Therapeutics: board membership, stock, and royalties.
Figures



References
-
- Ding C., Ren T. Near infrared fluorescent probes for detecting and imaging active small molecules. Coord. Chem. Rev. 2023;482:215080. doi: 10.1016/j.ccr.2023.215080. - DOI
-
- Yin H., Jiang W., Liu Y., Zhang D., Wu F., Zhang Y., Li C., Chen G., Wang Q. Advanced near-infrared light approaches for neuroimaging and neuromodulation. BMEMat. 2023;1:e12023. doi: 10.1002/bmm2.12023. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

