Development of a Novel Biomarker for the Progression of Idiopathic Pulmonary Fibrosis
- PMID: 38203769
- PMCID: PMC10779374
- DOI: 10.3390/ijms25010599
Development of a Novel Biomarker for the Progression of Idiopathic Pulmonary Fibrosis
Abstract
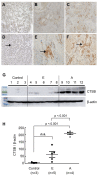
The progression of idiopathic pulmonary fibrosis (IPF) is diverse and unpredictable. We identified and validated a new biomarker for IPF progression. To identify a candidate gene to predict progression, we assessed differentially expressed genes in patients with advanced IPF compared with early IPF and controls in three lung sample cohorts. Candidate gene expression was confirmed using immunohistochemistry and Western blotting of lung tissue samples from an independent IPF clinical cohort. Biomarker potential was assessed using an enzyme-linked immunosorbent assay of serum samples from the retrospective validation cohort. We verified that the final candidate gene reflected the progression of IPF in a prospective validation cohort. In the RNA-seq comparative analysis of lung tissues, CD276, COL7A1, CTSB, GLI2, PIK3R2, PRAF2, IGF2BP3, and NUPR1 were up-regulated, and ADAMTS8 was down-regulated in the samples of advanced IPF. Only CTSB showed significant differences in expression based on Western blotting (n = 12; p < 0.001) and immunohistochemistry between the three groups of the independent IPF cohort. In the retrospective validation cohort (n = 78), serum CTSB levels were higher in the progressive group (n = 25) than in the control (n = 29, mean 7.37 ng/mL vs. 2.70 ng/mL, p < 0.001) and nonprogressive groups (n = 24, mean 7.37 ng/mL vs. 2.56 ng/mL, p < 0.001). In the prospective validation cohort (n = 129), serum CTSB levels were higher in the progressive group than in the nonprogressive group (mean 8.30 ng/mL vs. 3.00 ng/mL, p < 0.001). After adjusting for baseline FVC, we found that CTSB was independently associated with IPF progression (adjusted OR = 2.61, p < 0.001). Serum CTSB levels significantly predicted IPF progression (AUC = 0.944, p < 0.001). Serum CTSB level significantly distinguished the progression of IPF from the non-progression of IPF or healthy control.
Keywords: IPF; cathepsin; prediction; prognosis; progression.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Discovery and validation of extracellular/circulating microRNAs during idiopathic pulmonary fibrosis disease progression.Gene. 2015 May 10;562(1):138-44. doi: 10.1016/j.gene.2015.02.065. Epub 2015 Feb 25. Gene. 2015. PMID: 25725128
-
Gremlin-1 for the Differential Diagnosis of Idiopathic Pulmonary Fibrosis Versus Other Interstitial Lung Diseases: A Clinical and Pathophysiological Analysis.Lung. 2021 Jun;199(3):289-298. doi: 10.1007/s00408-021-00440-y. Epub 2021 Mar 26. Lung. 2021. PMID: 33770226 Free PMC article.
-
Integrated plasma proteomics and lung transcriptomics reveal novel biomarkers in idiopathic pulmonary fibrosis.Respir Res. 2021 Oct 24;22(1):273. doi: 10.1186/s12931-021-01860-3. Respir Res. 2021. PMID: 34689792 Free PMC article.
-
Potential biomarkers for diagnosis and disease evaluation of idiopathic pulmonary fibrosis.Chin Med J (Engl). 2023 Jun 5;136(11):1278-1290. doi: 10.1097/CM9.0000000000002171. Chin Med J (Engl). 2023. PMID: 37130223 Free PMC article. Review.
-
Biomarkers in idiopathic pulmonary fibrosis.Matrix Biol. 2018 Aug;68-69:404-421. doi: 10.1016/j.matbio.2018.01.023. Epub 2018 Jan 31. Matrix Biol. 2018. PMID: 29408012 Review.
Cited by
-
Bleomycin-Induced Pulmonary Fibrosis in Transgenic Mice Carrying the Human MUC5B rs35705950 Variant.Cells. 2024 Sep 11;13(18):1523. doi: 10.3390/cells13181523. Cells. 2024. PMID: 39329706 Free PMC article.
-
An Overview of the Role of Genetic factors in Idiopathic Pulmonary Fibrosis: Insights from Epidemiology to Prognosis.Int J Med Sci. 2025 Jun 12;22(12):2992-3006. doi: 10.7150/ijms.113226. eCollection 2025. Int J Med Sci. 2025. PMID: 40657392 Free PMC article. Review.
-
Elucidating the causal associations and mechanisms between circulating immune cells and idiopathic pulmonary fibrosis: new insights from Mendelian randomization and transcriptomics.Front Immunol. 2025 Jan 17;15:1437984. doi: 10.3389/fimmu.2024.1437984. eCollection 2024. Front Immunol. 2025. PMID: 39896814 Free PMC article.
References
-
- Azuma A., Taguchi Y., Ogura T., Ebina M., Taniguchi H., Kondoh Y., Suga M., Takahashi H., Nakata K., Sato A., et al. Exploratory analysis of a phase III trial of pirfenidone identifies a subpopulation of patients with idiopathic pulmonary fibrosis as benefiting from treatment. Respir. Res. 2011;12:143. doi: 10.1186/1465-9921-12-143. - DOI - PMC - PubMed
-
- Maher T.M., Molina-Molina M., Russell A.M., Bonella F., Jouneau S., Ripamonti E., Axmann J., Vancheri C. Unmet needs in the treatment of idiopathic pulmonary fibrosis-insights from patient chart review in five European countries. BMC Pulm. Med. 2017;17:124. doi: 10.1186/s12890-017-0468-5. - DOI - PMC - PubMed
-
- Raghu G., Collard H.R., Egan J.J., Martinez F.J., Behr J., Brown K.K., Colby T.V., Cordier J.-F., Flaherty K.R., Lasky J.A., et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

