Propolis Protects GC-1spg Spermatogonial Cells against Tert-Butyl Hydroperoxide-Induced Oxidative Damage
- PMID: 38203785
- PMCID: PMC10779084
- DOI: 10.3390/ijms25010614
Propolis Protects GC-1spg Spermatogonial Cells against Tert-Butyl Hydroperoxide-Induced Oxidative Damage
Abstract
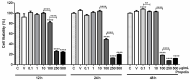
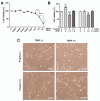
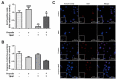
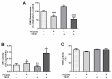
Propolis is a natural resin produced by honeybees with plenty of pharmacologic properties, including antioxidant activity. Oxidative stress disrupts germ cell development and sperm function, with demonstrated harmful effects on male reproduction. Several natural antioxidants have been shown to reduce oxidative damage and increase sperm fertility potential; however, little is known about the effects of propolis. This work evaluated the role of propolis in protecting spermatogonial cells from oxidative damage. Propolis' phytochemical composition and antioxidant potential were determined, and mouse GC-1spg spermatogonial cells were treated with 0.1-500 µg/mL propolis (12-48 h) in the presence or absence of an oxidant stimulus (tert-butyl hydroperoxide, TBHP, 0.005-3.6 µg/mL, 12 h). Cytotoxicity was assessed by MTT assays and proliferation by Ki-67 immunocytochemistry. Apoptosis, reactive oxygen species (ROS), and antioxidant defenses were evaluated colorimetrically. Propolis presented high phenolic and flavonoid content and moderate antioxidant activity, increasing the viability of GC-1spg cells and counteracting TBHP's effects on viability and proliferation. Additionally, propolis reduced ROS levels in GC-1spg, regardless of the presence of TBHP. Propolis decreased caspase-3 and increased glutathione peroxidase activity in TBHP-treated GC-1spg cells. The present study shows the protective action of propolis against oxidative damage in spermatogonia, opening the possibility of exploiting its benefits to male fertility.
Keywords: antioxidant; oxidative stress; propolis; spermatogonial cells; tert-butyl hydroperoxide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Regucalcin counteracts tert-butyl hydroperoxide and cadmium-induced oxidative stress in rat testis.J Appl Toxicol. 2017 Feb;37(2):159-166. doi: 10.1002/jat.3333. Epub 2016 Apr 25. J Appl Toxicol. 2017. PMID: 27109168
-
Aqueous extract of Terminalia arjuna attenuates tert-butyl hydroperoxide-induced oxidative stress in HepG2 cell model.Cell Biochem Funct. 2013 Mar;31(2):129-35. doi: 10.1002/cbf.2867. Epub 2012 Sep 7. Cell Biochem Funct. 2013. PMID: 22961563
-
Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines.Biochem Pharmacol. 2005 Dec 5;70(12):1796-806. doi: 10.1016/j.bcp.2005.08.022. Epub 2005 Oct 10. Biochem Pharmacol. 2005. PMID: 16216225
-
Effects of propolis intake on endurance exercise and molecular signaling related to inflammation and oxidative stress.Front Nutr. 2025 Feb 26;12:1539701. doi: 10.3389/fnut.2025.1539701. eCollection 2025. Front Nutr. 2025. PMID: 40078415 Free PMC article. Review.
-
Exploring the Prospective Role of Propolis in Modifying Aging Hallmarks.Cells. 2024 Feb 24;13(5):390. doi: 10.3390/cells13050390. Cells. 2024. PMID: 38474354 Free PMC article. Review.
Cited by
-
Hydrogen Peroxide-Releasing Hydrogel-Mediated Cellular Senescence Model for Aging Research.Biomater Res. 2025 Mar 14;29:0161. doi: 10.34133/bmr.0161. eCollection 2025. Biomater Res. 2025. PMID: 40092651 Free PMC article.
-
Propolis extract nanoparticles alleviate diabetes-induced reproductive dysfunction in male rats: antidiabetic, antioxidant, and steroidogenesis modulatory role.Sci Rep. 2024 Dec 23;14(1):30607. doi: 10.1038/s41598-024-81949-z. Sci Rep. 2024. PMID: 39715797 Free PMC article.
-
Homotherapy for heteropathy of chronic kidney disease and oligoasthenozoospermia through regulating SIRT1/NF-κB pathway by Shenqi pills.Front Pharmacol. 2025 Jun 9;16:1551423. doi: 10.3389/fphar.2025.1551423. eCollection 2025. Front Pharmacol. 2025. PMID: 40552151 Free PMC article.
-
Targeting novel regulated cell death: disulfidptosis in cancer immunotherapy with immune checkpoint inhibitors.Biomark Res. 2025 Feb 26;13(1):35. doi: 10.1186/s40364-025-00748-4. Biomark Res. 2025. PMID: 40012016 Free PMC article. Review.
References
-
- Hallajzadeh J., Milajerdi A., Amirani E., Attari V.E., Maghsoudi H., Mirhashemi S.M. Effects of propolis supplementation on glycemic status, lipid profiles, inflammation and oxidative stress, liver enzymes, and body weight: A systematic review and meta-analysis of randomized controlled clinical trials. J. Diabetes Metab. Disord. 2021;20:831–843. doi: 10.1007/s40200-020-00696-w. - DOI - PMC - PubMed
-
- Bankova V., Dyulgerov A., Popov S., Evstatieva L., Kuleva L., Pureb O., Zamjansan Z. Propolis produced in Bulgaria and Mongolia: Phenolic compounds and plant origin. Apidologie. 1992;23:79–85. doi: 10.1051/apido:19920109. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

