Nanoparticle-Encapsulated Epirubicin Efficacy in the Inhibition of Growth of Orthotopic Ovarian Patient-Derived Xenograft in Immunocompromised Mice
- PMID: 38203818
- PMCID: PMC10779551
- DOI: 10.3390/ijms25010645
Nanoparticle-Encapsulated Epirubicin Efficacy in the Inhibition of Growth of Orthotopic Ovarian Patient-Derived Xenograft in Immunocompromised Mice
Abstract
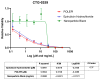
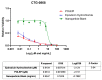
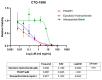
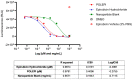
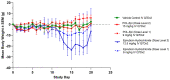
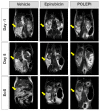
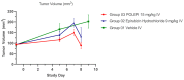
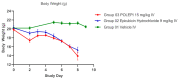
Epirubicin hydrochloride (EPI) is an anticancer drug widely used in the treatment of many solid tumors, including ovarian cancer. Because of its anatomical location, ovarian cancer shows symptoms when it is already in an advanced stage and is thus more difficult to treat. Epirubicin hydrochloride kills cancer cells effectively, but its dose escalation is limited by its severe toxicity. By encapsulating epirubicin in dextran-based nanoparticles (POLEPI), we expected to deliver higher and thus clinically more effective doses directly to tumors, where epirubicin would be released and retained longer in the tumor. The antitumor activity of POLEPI compared to EPI was first tested ex vivo in a series of ovarian cancer patient-derived tumor xenografts (PDX). The most promising PDX was then implanted orthotopically into immunocompromised mice, and tumor growth was monitored via magnetic resonance imaging (MRI). Although we succeeded in suppressing the growth of ovarian cancer derived from a patient, in a mouse model by 70% compared to 40% via EPI in 5 days after only one injection, we could not eliminate serious side effects, and the study was terminated prematurely for humane reasons.
Keywords: epirubicin hydrochloride; nanoparticles; orthotopic ovarian cancer model; ovarian cancer; patient-derived tumor xenografts (PDX).
Conflict of interest statement
Authors declare conflicts of interest. All authors (except Adam Kiciak) are employed by the NanoVelos S.A. company which is developing the nanoparticle-encapsulated epirubicin (POLEPI). Adam Kiciak and Tomasz Ciach are members of NanoGroup S.A. However, they also declare that this situation did not have any influence on the solidity and veracity of the presented results.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

