The landscape of nanoparticle-based siRNA delivery and therapeutic development
- PMID: 38204162
- PMCID: PMC10861989
- DOI: 10.1016/j.ymthe.2024.01.005
The landscape of nanoparticle-based siRNA delivery and therapeutic development
Abstract
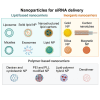
Five small interfering RNA (siRNA)-based therapeutics have been approved by the Food and Drug Administration (FDA), namely patisiran, givosiran, lumasiran, inclisiran, and vutrisiran. Besides, siRNA delivery to the target site without toxicity is a big challenge for researchers, and naked-siRNA delivery possesses several challenges, including membrane impermeability, enzymatic degradation, mononuclear phagocyte system (MPS) entrapment, fast renal excretion, endosomal escape, and off-target effects. The siRNA therapeutics can silence any disease-specific gene, but their intracellular and extracellular barriers limit their clinical applications. For this purpose, several modifications have been employed to siRNA for better transfection efficiency. Still, there is a quest for better delivery systems for siRNA delivery to the target site. In recent years, nanoparticles have shown promising results in siRNA delivery with minimum toxicity and off-target effects. Patisiran is a lipid nanoparticle (LNP)-based siRNA formulation for treating hereditary transthyretin-mediated amyloidosis that ultimately warrants the use of nanoparticles from different classes, especially lipid-based nanoparticles. These nanoparticles may belong to different categories, including lipid-based, polymer-based, and inorganic nanoparticles. This review briefly discusses the lipid, polymer, and inorganic nanoparticles and their sub-types for siRNA delivery. Finally, several clinical trials related to siRNA therapeutics are addressed, followed by the future prospects and conclusions.
Keywords: RNA interference; clinical trials; inorganic carrier; lipid nanoparticle; polymer; siRNA.
Copyright © 2024 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Similar articles
-
Lipid Nanoparticle Technology for Clinical Translation of siRNA Therapeutics.Acc Chem Res. 2019 Sep 17;52(9):2435-2444. doi: 10.1021/acs.accounts.9b00368. Epub 2019 Aug 9. Acc Chem Res. 2019. PMID: 31397996 Review.
-
Lipid Nanoparticles Enabling Gene Therapies: From Concepts to Clinical Utility.Nucleic Acid Ther. 2018 Jun;28(3):146-157. doi: 10.1089/nat.2018.0721. Epub 2018 Apr 23. Nucleic Acid Ther. 2018. PMID: 29683383 Review.
-
Leading RNA Interference Therapeutics Part 1: Silencing Hereditary Transthyretin Amyloidosis, with a Focus on Patisiran.Mol Diagn Ther. 2020 Feb;24(1):49-59. doi: 10.1007/s40291-019-00434-w. Mol Diagn Ther. 2020. PMID: 31701435 Review.
-
Recent Advances in Engineering Carriers for siRNA Delivery.Macromol Biosci. 2024 Apr;24(4):e2300362. doi: 10.1002/mabi.202300362. Epub 2024 Jan 12. Macromol Biosci. 2024. PMID: 38150293 Review.
-
Enhancing siRNA cancer therapy: Multifaceted strategies with lipid and polymer-based carrier systems.Int J Pharm. 2024 Sep 30;663:124545. doi: 10.1016/j.ijpharm.2024.124545. Epub 2024 Aug 2. Int J Pharm. 2024. PMID: 39098747 Review.
Cited by
-
siRNA Therapeutics: From Bench Lab. to Clinics.Pharmaceuticals (Basel). 2024 Mar 26;17(4):416. doi: 10.3390/ph17040416. Pharmaceuticals (Basel). 2024. PMID: 38675378 Free PMC article.
-
Antibody/siRNA Nanocarriers Against Wnt Signaling Suppress Oncogenic and Stem-Like Behavior in Triple-Negative Breast Cancer Cells.J Biomed Mater Res A. 2025 Jan;113(1):e37867. doi: 10.1002/jbm.a.37867. J Biomed Mater Res A. 2025. PMID: 39760151
-
ROS-Responsive Ferrocenyl Amphiphilic PAMAM Dendrimers for On-Demand Delivery of siRNA Therapeutics to Cancer Cells.Pharmaceutics. 2024 Jul 13;16(7):936. doi: 10.3390/pharmaceutics16070936. Pharmaceutics. 2024. PMID: 39065632 Free PMC article.
-
Metabolic Stability and Targeted Delivery of Oligonucleotides: Advancing RNA Therapeutics Beyond The Liver.J Med Chem. 2025 Apr 10;68(7):6870-6896. doi: 10.1021/acs.jmedchem.4c02528. Epub 2025 Jan 8. J Med Chem. 2025. PMID: 39772535 Free PMC article. Review.
-
Dysregulation of GAS5-miRNA-Mediated Signaling Pathways in Cancer Pathobiology: A Comprehensive Exploration of Pathways Influenced by this Axis.Biochem Genet. 2025 Apr;63(2):1149-1175. doi: 10.1007/s10528-024-10997-x. Epub 2024 Dec 24. Biochem Genet. 2025. PMID: 39718723 Review.
References
-
- Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
-
- Elbashir S.M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. - PubMed
-
- Saw P.E., Song E.W. siRNA therapeutics: a clinical reality. Sci. China. Life Sci. 2020;63:485–500. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

