Poliovirus serological assay after the cVDPV1 outbreak in Papua New Guinea: a cross-sectional study from 2020 to 2021
- PMID: 38204497
- PMCID: PMC10777103
- DOI: 10.1016/j.lanwpc.2023.100986
Poliovirus serological assay after the cVDPV1 outbreak in Papua New Guinea: a cross-sectional study from 2020 to 2021
Abstract
Background: In June 2018, a type 1 circulating vaccine-derived poliovirus (cVDPV1) outbreak was declared in Papua New Guinea (PNG), resulting in a total of 26 paralytic confirmed cases. Eight vaccination campaign rounds with bivalent oral poliovirus vaccine (bOPV) were carried out in response. Prevalence of neutralizing polio antibodies in children was assessed two years after the outbreak response was completed.
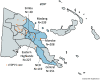
Methods: We conducted a cross-sectional serological survey among children aged 6 months-10 years selected from six provinces in PNG to evaluate seroprevalence of neutralizing polio antibodies to the three poliovirus serotypes and analyse sociodemographic risk factors.
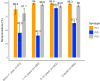
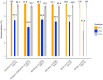
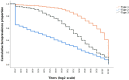
Findings: We included 984 of 1006 enrolled children in the final analysis. The seroprevalence of neutralizing polio antibodies for serotype 1, 2 and 3 was 98.3% (95% CI: 97.4-98.9), 63.1% (95% CI: 60.1-66.1) and 95.0% (95% CI: 93.6-96.3), respectively. Children <1 year had significantly lower type 1 seroprevalence compared to older children (p < 0.001); there were no significant differences in seroprevalence among provinces.
Interpretation: PNG successfully interrupted transmission of cVDPV1 with several high coverage bOPV campaigns and seroprevalence remained high after two years. The emergence of cVDPV strains underscores the importance of maintaining high levels of routine immunization coverage and effective surveillance systems for early detection.
Funding: World Health Organization through a Rotary International IPPC grant.
Keywords: Bivalent oral poliovirus vaccine; Outbreak response; Papua New Guinea; Polio; Seroprevalence; Vaccine-derived poliovirus.
© 2023 Published by Elsevier Ltd.
Conflict of interest statement
All authors—no conflict of interest declared.
Figures
Similar articles
-
Responding to a cVDPV1 outbreak in Ukraine: Implications, challenges and opportunities.Vaccine. 2017 Aug 24;35(36):4769-4776. doi: 10.1016/j.vaccine.2017.04.036. Epub 2017 May 19. Vaccine. 2017. PMID: 28528761 Free PMC article. Review.
-
Poliovirus antibodies following two rounds of campaigns with a type 2 novel oral poliovirus vaccine in Liberia: a clustered, population-based seroprevalence survey.Lancet Glob Health. 2023 Jun;11(6):e917-e923. doi: 10.1016/S2214-109X(23)00116-X. Lancet Glob Health. 2023. PMID: 37202026 Free PMC article.
-
Update on Vaccine-Derived Poliovirus Outbreaks - Worldwide, January 2021-December 2022.MMWR Morb Mortal Wkly Rep. 2023 Apr 7;72(14):366-371. doi: 10.15585/mmwr.mm7214a3. MMWR Morb Mortal Wkly Rep. 2023. PMID: 37022974 Free PMC article.
-
Assessment of serological responses following vaccination campaigns with type 2 novel oral polio vaccine: a population-based study in Tajikistan in 2021.Lancet Glob Health. 2022 Dec;10(12):e1807-e1814. doi: 10.1016/S2214-109X(22)00412-0. Lancet Glob Health. 2022. PMID: 36400086 Free PMC article.
-
Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis.Cochrane Database Syst Rev. 2019 Dec 5;12(12):CD011260. doi: 10.1002/14651858.CD011260.pub2. Cochrane Database Syst Rev. 2019. PMID: 31801180 Free PMC article.
Cited by
-
Impact of novel Oral Poliovirus Type 2 vaccination campaigns: A seroprevalence survey in Nigeria, 2022.Vaccine. 2025 Apr 30;54:126978. doi: 10.1016/j.vaccine.2025.126978. Epub 2025 Mar 19. Vaccine. 2025. PMID: 40112661 Free PMC article. No abstract available.
References
-
- WHO . Handbook of resolutions and decisions of the World Health Assembly and the Executive Board, Volume III, 3rd edition (1985–1992) WHO; Geneva: 1993. Resolution WHA. 41.28. Global eradication of poliomyelitis by the year 2000.
-
- WHO, GPEI . 2018. Polio post-certification strategy.http://polioeradication.org/polio-today/preparing-for-a-polio-free-world...
-
- GPEI, WHO . 2019. Fact sheet: vaccine-derived poliovirus.https://polioeradication.org/wp-content/uploads/2018/07/GPEI-cVDPV-Fact-...
-
- WHO . 2023. Global wild poliovirus 2017 - 2023.https://polioeradication.org/polio-today/polio-now/wild-poliovirus-list/
-
- WHO . 2023. Global circulating vaccine-derived poliovirus (cVDPV) as of 09 May 2023.https://polioeradication.org/this-week/circulating-vaccine-derived-polio...
Grants and funding
LinkOut - more resources
Full Text Sources

