Influenza vaccine effectiveness in Europe: Results from the 2022-2023 VEBIS (Vaccine Effectiveness, Burden and Impact Studies) primary care multicentre study
- PMID: 38204584
- PMCID: PMC10777262
- DOI: 10.1111/irv.13243
Influenza vaccine effectiveness in Europe: Results from the 2022-2023 VEBIS (Vaccine Effectiveness, Burden and Impact Studies) primary care multicentre study
Abstract
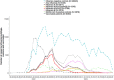
Background: Influenza A(H3N2) viruses dominated early in the 2022-2023 influenza season in Europe, followed by higher circulation of influenza A(H1N1)pdm09 and B viruses. The VEBIS primary care network estimated the influenza vaccine effectiveness (VE) using a multicentre test-negative study.
Materials and methods: Primary care practitioners collected information and specimens from patients consulting with acute respiratory infection. We measured VE against any influenza, influenza (sub)type and clade, by age group, by influenza vaccine target group and by time since vaccination, using logistic regression.
Results: We included 38 058 patients, of which 3786 were influenza A(H3N2), 1548 influenza A(H1N1)pdm09 and 3275 influenza B cases. Against influenza A(H3N2), VE was 36% (95% CI: 25-45) among all ages and ranged between 30% and 52% by age group and target group. VE against influenza A(H3N2) clade 2b was 38% (95% CI: 25-49). Overall, VE against influenza A(H1N1)pdm09 was 46% (95% CI: 35-56) and ranged between 29% and 59% by age group and target group. VE against influenza A(H1N1)pdm09 clade 5a.2a was 56% (95% CI: 46-65) and 79% (95% CI: 64-88) against clade 5a.2a.1. VE against influenza B was 76% (95% CI: 70-81); overall, 84%, 72% and 71% were among 0-14-year-olds, 15-64-year-olds and those in the influenza vaccination target group, respectively. VE against influenza B with a position 197 mutation of the hemagglutinin (HA) gene was 79% (95% CI: 73-85) and 90% (95% CI: 85-94) without this mutation.
Conclusion: The 2022-2023 end-of-season results from the VEBIS network at primary care level showed high VE among children and against influenza B, with lower VE against influenza A(H1N1)pdm09 and A(H3N2).
Keywords: Europe; influenza; influenza vaccine; multicentre study; vaccine effectiveness.
© 2024 The Authors. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
None.
Figures

References
-
- Recommended Composition of Influenza Virus Vaccines for Use in the 2022–2023 Northern Hemisphere Influenza Season. Available from: https://www.who.int/publications/m/item/recommended-composition-of-influ...
-
- Flu News Europe . Flu news Europe. Available from: https://flunewseurope.org/SeasonOverview
-
- Valenciano M, Kissling E, Cohen JM, et al. Estimates of pandemic influenza vaccine effectiveness in Europe, 2009–2010: results of influenza monitoring vaccine effectiveness in Europe (I‐MOVE) multicentre case‐control study. PLoS Med. 2011;8(1):e1000388. doi: 10.1371/journal.pmed.1000388 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

