A comprehensive review of discovery and development of drugs discovered from 2020-2022
- PMID: 38204591
- PMCID: PMC10777120
- DOI: 10.1016/j.jsps.2023.101913
A comprehensive review of discovery and development of drugs discovered from 2020-2022
Abstract
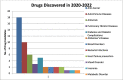
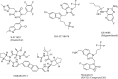
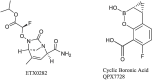
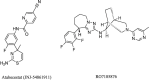
To fully evaluate and define the new drug molecule for its pharmacological characteristics and toxicity profile, pre-clinical and clinical studies are conducted as part of the drug research and development process. The average time required for all drug development processes to finish various regulatory evaluations ranges from 11.4 to 13.5 years, and the expense of drug development is rising quickly. The development in the discovery of newer novel treatments is, however, largely due to the growing need for new medications. Methods to identify Hits and discovery of lead compounds along with pre-clinical studies have advanced, and one example is the introduction of computer-aided drug design (CADD), which has greatly shortened the time needed for the drug to go through the drug discovery phases. The pharmaceutical industry will hopefully be able to address the present and future issues and will continue to produce novel molecular entities (NMEs) to satisfy the expanding unmet medical requirements of the patients as the success rate of the drug development processes is increasing. Several heterocyclic moieties have been developed and tested against many targets and proved to be very effective. In-depth discussion of the drug design approaches of newly found drugs from 2020 to 2022, including their pharmacokinetic and pharmacodynamic profiles and in-vitro and in-vivo assessments, is the main goal of this review. Considering the many stages these drugs are going through in their clinical trials, this investigation is especially pertinent. It should be noted that synthetic strategies are not discussed in this review; instead, they will be in a future publication.
Keywords: CADD; Characterize; Clinical candidates; Drug discovery and development; NMEs; Unmet medical needs.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
[Aiming for zero blindness].Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):168-93; discussion 194. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854109 Review. Japanese.
-
Alternative strategies in drug development: clinical pharmacological aspects.Int J Clin Pharmacol Ther. 1999 Dec;37(12):575-83. Int J Clin Pharmacol Ther. 1999. PMID: 10599949 Review.
-
Traditional and Novel Computer-Aided Drug Design (CADD) Approaches in the Anticancer Drug Discovery Process.Curr Cancer Drug Targets. 2023;23(5):333-345. doi: 10.2174/1568009622666220705104249. Curr Cancer Drug Targets. 2023. PMID: 35792126 Review.
Cited by
-
Chemical composition and toxicity studies on Lantana camara L. flower essential oil and its in silico binding and pharmacokinetics to superoxide dismutase 1 for amyotrophic lateral sclerosis (ALS) therapy.RSC Adv. 2024 Aug 5;14(33):24250-24264. doi: 10.1039/d4ra04281f. eCollection 2024 Jul 26. RSC Adv. 2024. PMID: 39104562 Free PMC article.
-
National Granted Programs and Public Funds for Biopharmaceutical Startups in Japan.Pharmacol Res Perspect. 2025 Jun;13(3):e70106. doi: 10.1002/prp2.70106. Pharmacol Res Perspect. 2025. PMID: 40289412 Free PMC article. Review.
References
-
- Angst D., Gessier F., Janser P., Vulpetti A., Wälchli R., Beerli C., et al. Discovery of LOU064 (Remibrutinib), a potent and highly selective covalent inhibitor of Bruton’s Tyrosine Kinase. J. Med. Chem. 2020;63(10):5102–5118. - PubMed
-
- Asahina Y., Wurtz N.R., Arakawa K., Carson N., Fujii K., Fukuchi K., et al. Discovery of BMS-986235/LAR-1219: a potent formyl peptide receptor 2 (FPR2) selective agonist for the prevention of heart failure. J. Med. Chem. 2020;63(17):9003–9019. - PubMed
-
- Berger M., May E., Rehwinkel H., Schäcke H., Neuhaus R., Rottmann A., et al. Discovery of the potent non-steroidal glucocorticoid receptor modulator BAY 1003803 as clinical candidate. Bioorg. Med. Chem. Lett. 2020;30(16) - PubMed
-
- Brandao D.J., Fontenelle L.F., da Silva S.A., Menezes P.R., Pastor-Valero M. Depression and excess mortality in the elderly living in low-and middle-income countries: systematic review and meta-analysis. Int. J. Geriatr. Psychiatry. 2019;34(1):22–30. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

